December 19, 2019 – The following posting is inspired by a recent Peter Diamandis e-mail blast in which he discusses the changes happening in biopharmacology because of the proliferation of information in the form of massive amounts of data and new technologies. Among these innovations were new ways of applying artificial intelligence (AI) to the development of new pharmaceuticals and our uncovering of new nanoscience and a better understanding of the role of proteins in finding cures for many of the most intractable diseases. So without further ado, I will use some of Peter’s words along with some additions from me to explain.
GANs and New Pharmaceuticals
Around 2012, a computer scientist-turned-biophysicist, Alex Zhavoronkov, noticed that AI was getting better at image, voice, and text recognition. He knew that all three tasks shared a critical commonality, massive datasets available to use to train the AI. By 2014, Zhavoronkov was using the technology for discovering new drugs.
Zhavoronkov had heard about a new AI technique known as generative adversarial networks (GANs). How does it work? By pitting two neural networks against each other, with minimal instructions the technology could produce novel outcomes. Before this researchers were using GANs to do things like design new objects or create one-of-a-kind, fake human faces. It was Zhavoronkov who saw pharmacological possibilities. He figured GANs would allow researchers to verbally describe drug attributes in terms such as “The compound should inhibit protein X at concentration Y with minimal side effects in humans,” and then let the AI construct an appropriate molecule from scratch.
Zhavoronkov set up Insilico Medicine on the campus of Johns Hopkins University in Baltimore, Maryland, and over three years using GANs was able to develop a drug discovery engine that sifts through millions of data samples and determines the biological signature and characteristics of specific diseases. It can then identify the most promising treatment targets and generate molecules perfectly suited for them. States Zhavoronkov, “The result is an explosion in potential drug targets and a much more efficient testing process … AI allows us to do with fifty people what a typical drug company does with five thousand.”
Right now, the GANS is being used to find new drugs for cancer, aging, fibrosis, Parkinson’s, Alzheimer’s, ALS, diabetes, and many others. The first drug coming to market is a treatment for hair loss slated to start Phase I trials in 2020.
Insilico is also in the early stages of using its tools to predict the outcomes of clinical trials in advance. If successful, the technique will enable researchers to shorten the time and money spent in the traditional testing process.
Applying AI to Protein Folding
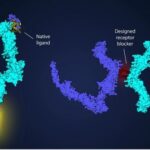
For those of you unfamiliar with the role proteins play in our bodies I refer you to an article posted on this site back in 2013 and a game called FoldIt, created by researchers at the University of Washington, which uses the collective effort of crowdsourcing to develop new protein folds. And you may not be aware that CRISPR/Cas9, the gene-editing tool taking genetic engineering mainstream, is in fact, a tool that uses a protein to excise bad genes and replace them with healthy ones. So proteins are a hot subject in the world of pharmacology today and scientists are using AI to identify new drug targets that bind with them.
Proteins consist of amino acids that fold in different ways to take on different functions. A protein containing a hundred amino acids, rather small by body standards, produces a googol-cubed-worth of potential shapes. (That’s a one followed by three hundred zeroes.)
Back in 1994, a biannual protein-folding competition was created with little success until 2018 when the creators of DeepMind turned neural networks loose on the problem. DeepMind took on the task of determining the most likely distance between a protein’s base pairs and the angles of their chemical bonds. They called their end-product, AlphaFold. In its first entry into the competition, it solved 25 of 43 protein-folding problems compared to the second-place finisher with only three.
How does AlphaFold work? It uses two neural networks, one focused on predicting distances between pairs of specific amino acids, and a second evaluating the possible angles of the chemical bonds between them. The two return a score estimating likely structures that match a compilation of known protein fragments, and the generation of completely new protein structures.
The potential implications of AI-accelerated protein folding for new pharma cannot be underestimated and what it could mean for treating patients with Huntington’s, ALS, myotonic dystrophy, and other diseases.
Targeted Drug Delivery Using Gene-Editors and Nanobots
Another theatre of war for improved drugs is in the realm of drug delivery. Converging exponential technologies are paving the way for massive implications in both human health and industry shifts. The aforementioned CRISPR stands to revolutionize synthetic biology and the treatment of genetically-linked diseases. Researchers are now demonstrating how this gene-editing tool can be applied to create materials that shape-shift on command. Think of the potential of materials that can dissolve instantaneously when stimulated, releasing a specified drug in a highly targeted location.
Then there are nanobots, micro-machines capable of providing targeted drug delivery. In a recent review of medical micro- and nanorobotics, the lead authors from the University of Texas at Austin and the University of California, San Diego found numerous successful tests of in vivo operations using medical micro- and nanorobots.
Drugs From The Future
Inefficient, slow-to-innovate, and risk-averse industries will be disrupted in the years ahead and Big Pharma is an area worth watching right now. Two areas, in particular, are getting headlines, advances in addressing longevity, and in disease prevention. The aforementioned Insilico is one of the companies leading the way.
The convergence of massive datasets, growing computational power, the rise of quantum computing, and innovations in AI, are bringing us closer to a world in which personalized drugs delivered directly to specified targets will become the standard of care. Rejuvenation biotechnology will be commercially available sooner than you think.
Zhavoronkov sees the timeline at “maybe 20 years … a reasonable horizon for tangible rejuvenational biotechnology.”
How might any of us use an extra 20 or more healthy years in our lives? What impact would that have on the world?