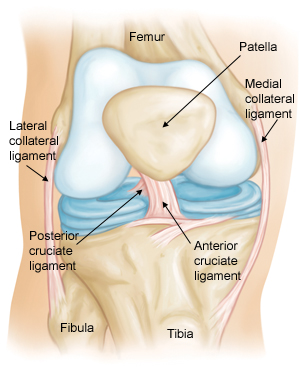
May 8, 2015 – A close friend of mine this week told me he is going in for knee replacement surgery next month. Also known as arthroplasty it involves replacing the knee joint (seen in the image below) with artificial materials. Three years ago I was advised to do the same but have chosen to wait. My friend is raising two young children and keeping up has put a lot of wear and tear on his knees to the point that he chose a surgical option. I, on the other hand am waiting.
My problem stems back to an injury when I was 20. I broke both of my legs and although I had multiple surgeries my left leg twisted slightly during the healing process putting extra strain on that knee. By age 25 I was already experiencing the first signs of osteoarthritis and I have progressively watched it worsen over the years. Today I have no cartilage remaining in my left knee and manage the pain using an occasional Celebrex. The stress on my left knee has also impacted the one on the right with some cartilage loss, but nothing like what I am experiencing on my left side.
As I said before rather than invasive knee joint replacement I am waiting for advances in stem cell therapy and 3D printing to avoid surgery. Instead I’m reading the latest journal papers describing therapies being tested in small clinical trials like one recently described by researchers at Tehran University using mesenchymal stem cell transplants to reverse osteoarthritis.
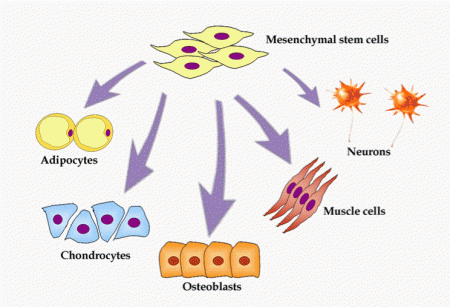
What are mesenchymal stem cells? Also known as multipotent stromal cells or MSCs, they can differentiate into osteoblasts (bone), chondrocytes (cartilage), myocytes (muscle), adipocytes (fat) and neurons. They are typcially found in bone marrow or in umbilical cord blood and are self-renewing. As you can see from above their ability to differentiate is extraordinary. Thus they are excellent candidates for therapeutic repair and tissue regeneration.
In the case of the Tehran study four patients with knee osteoarthritis participated ranging in age from 55 to 65. All had moderate to severe osteoarthritis. Each consented to having 30 milliliters of bone marrow extracted. The bone marrow was cultured to grow MSCs. After 4 to 5 weeks the MSCs were injected into one of the knees of each patient.
The result for 3 of the 4 was the lessening of joint pain. All were able to climb stairs and experience less pain. All experienced less knee joint grinding and cracking. Range of motion was minimally impacted. The clinical study conclusion was encouraging but not excellent.
Regenexx, an American company located in Denver, Colorado, is doing research involving stem cell and minimally invasive joint repair similar to the clinical trial done in Tehran. Regenexx is seeking 50 candidates between ages 18 and 70 for their knee osteoarthritis study. Half will undergo stem cell therapy treatment while the other half will receive knee osteoarthritis exercise therapy. Those in the exercise group will be able to cross over to receive stem cell treatment after 3 months. The Regenexx SD process appears very similar to the one described in the Tehran study with stem cells harvested from trial participants, concentrated in the lab and then reinjected into the damaged joint. 6 week, 3 month, 6 month, 12 month and 24 month post-injection follow ups are planned. End results will be compared against those undergoing arthroplasty.
There are enormous challenges in healing a damaged knee. The main one is caused by restricted blood flow to the joint. That’s why when athletes tear a knee ligament it can take a long time to repair. As a result they opt for surgery. I know from personal experience just how debilitating such tears can be and the length of time it takes for self healing. I partially tore my left medial collateral ligament three years ago while walking my dog. Because it was a partial tear my doctor advised me to let it self heal. It took more than a year.
I will be following the Regenexx clinical trial with great interest over the next two years to see if this presents me with an alternative to arthroplasty. If it does I’ll be glad I waited.