It seems that in the last few weeks I am writing about hydrogen more than at any time since starting this science and technology blog twelve years ago. Why hydrogen is suddenly in the news may have a lot to do with a growing sense of urgency for industry and governments to achieve net-zero emissions by mid-century in an effort to fight climate change.
Recently I came across a company spun out of Israel’s Technion that claims to have created a way to produce zero-emission green hydrogen using a methodology named E-TAC. Led by Dr. Hen Dotan, a material scientist and engineer, Professor Gideon Grader, advanced ceramic materials and process engineering specialist from the Technion, and Avner Rothschild, head of Electrochemical Materials and Devices research at the Technion and fellow of the National Academy of Sciences, the company, H2PRO, is focused on turning hydrogen into the ideal fuel for a decarbonized world in the 21st century.
Annual hydrogen consumption by industry today amounts to 70 million tons with almost all of it coming from grey hydrogen sources. In other words, hydrogen is produced from fossil fuels in a process that releases carbon dioxide (CO2) into the atmosphere, contributing to global warming.
Green hydrogen today is produced by electrolysis, splitting water (H2O) into its hydrogen and oxygen components. The energy needed comes from renewable sources like hydro, wind, and solar to be classified as green. The materials required include expensive membranes that frequently need replacement. And then there is the challenge of keeping the two gases separated. The complexity of this type of production has made green hydrogen cost-prohibitive when compared to other fuels like diesel, gasoline, or liquid natural gas (LNG).
H2PRO has invented a process called E-TAC with the acronym standing for a two-phase approach, the first Electrochemical, the second Thermally Activated Chemical (TAC).
The electrochemical phase involves a charged anode made of materials similar to the ones used by other electrolyzers. The TAC phase uses platinized nickel-coated stainless-steel mesh cathodes which when exposed to an alkaline water solution allow for the fast generation of hydrogen gas.
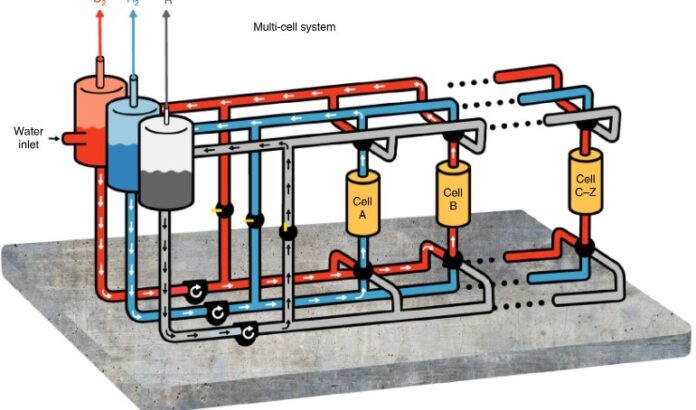
In the illustration that accompanies this posting (see above), the schematic shows a multi-cell E-TAC process with cold electrolyte (25 Celsius) circulating through cell A, generating hydrogen gas that subsequently is separated into its own storage tank seen in blue on the left. Cell B containing a hot electrolyte (95 Celsius) is used to produce oxygen which is also separated and stored (see the red tank on the left). Fluid in the grey tank circulates throughout the process helping to push the two gases to their respective storage tanks (the blue and red) without allowing mixing. The system when scaled for commercial production will be hermetically sealed so that high-purity hydrogen and oxygen streams can be produced safely.
What are the advantages of H2PRO’s invention?
- The process is done in nearly thermoneutral conditions which makes it eminently portable for use in many areas from transportation refueling stations, to fuel cell systems, to chemical synthesis for energy storage.
- E-TAC eliminates the need for a membrane the most expensive part of electrolyzers.
- Conversion efficiency is 98.7% from water to hydrogen and oxygen, much more efficient than the best-reported water oxidation technologies at 75%.
- The separation and storage of the gases with two different streams and processes removes potential hazards.
- High-pressure production without the use of compressors further reduces costs.
- Easy to scale and requiring less maintenance than existing water electrolysis processes makes E-TAC affordable and flexible.