July 24, 2018 – I have written a great deal about CRISPR and other gene therapies here at 21stcentech.com, but never about antisense. What is it? How does it differ from these other therapies that are increasingly making the headlines?
Antisense technology is designed to manipulate gene expression in cells. Using the analogy of writing and posting a letter, gene expression is what your genes write down in a letter which is then posted, where it is picked up for delivery by messenger RNA, our cell’s postman. The messenger RNA is not just the deliveryman, it is also the opener and reader of the letter. The content is in the form of specific instructions to use the material available to it to build a specific protein or molecule which then impacts the cell for a specific purpose.
Some genes express themselves from the moment of conception. Many others are on time delay switching on at different stages in our lives. Sometimes the genes we inherit are faulty and so they write down instructions that lead to bad outcomes in our bodies. That’s how children are born with malformed hearts. And that’s how children can appear to be born healthy and then later develop intractable diseases.
Antisense tools can do something about these imperfect genes by regulating the message being expressed, effectively silencing it before it leads to the creation of a protein that could have negative consequences. The technology employed is highly specific because it targets one gene at a time. And the technology varies depending on what is being treated? It could be cancer, or it could be any other genetically-caused disease.
In a story that appeared yesterday in MIT Technology Review, author Antonio Regalado describes how a scientific husband and wife team are trying to use antisense technology to stop a disease called fatal familial insomnia. The wife, Sonia Vallabh, inherited the gene from her mother who died from this disease, and so now the team is racing the clock to develop the antisense technology to defuse this genetic bomb in her body.
The cause of fatal familial insomnia is a defect in the PRNP gene found on the short arm of chromosome 20 in the nucleus of our cells. A genetic mutation produces incorrect instructions which lead to the messenger RNA executing an incorrectly folded protein designated PrP. PrP stands for prion protein which in cases of fatal familial insomnia is mishappen, impacting the thalamus, a section within our brain responsible for sleep and wake cycles, visual and auditory information processing, our sense of balance, how we experience pain, some aspects of learning, memory, speech and language understanding and emotional expression. Needless to say, the thalamus is a pretty important part of who we are.
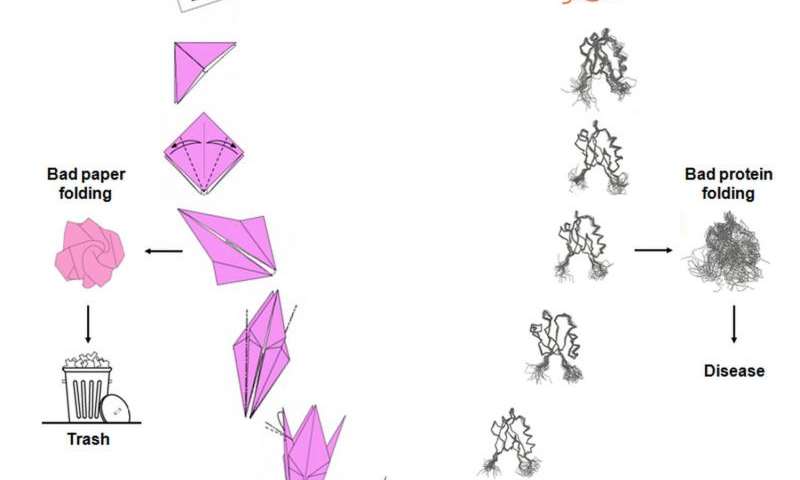
Bad protein folding is akin to bad origami (see illustration below). Instead of a perfect representation of an origami paper crane, you end up with mush. So it is with proteins that get misfolded except that unlike the one-off paper crane, the proteins perpetuate the misfolds making copies of themselves. In the case of PrP, this defective protein replicates itself acting as a toxin to neurons in the thalamus and leading to the death of brain cells important for human functions previously described.
Fatal familial insomnia is inherited from one parent. A single copy of the defective PRNP gene gets passed along leading to 50% chance that an offspring will receive it. After her mother’s diagnosis, Vallabh tested for the defective prion and found that she had it. Today she is 34 and has worked with her husband for the last seven years looking for a way to stop the defective prion from eventually killing her.
In the MIT article, Regalado states that the husband and wife team may have the answer in a mirror-image molecule which could be delivered to the thalamus to block the defective prion from killing neurons. They are working with the drug company, Ionis Pharmaceuticals, who recently developed an antisense treatment for the childhood neurological disorder, spinal muscular atrophy.
The missing delivery mechanism could be a retrovirus which would contain the blocking molecule. Injected as a serum into the spinal column a retroviral load would have to make its way to the brain where it could replicate and block the defective prion.
For Vallabh who has yet to show any symptoms of fatal familial insomnia, finding the delivery mechanism before the onset of the symptoms is critical. It could happen any time or twenty years from now. It is a ticking time bomb within her and motivation aplenty to continue the work that could be a template for other antisense therapies to cure many other prion-related diseases such as Creutzfelt-Jakob disease, known as mad cow, Parkinson’s, and Huntington’s.
Vallabh intends to be the first volunteer to try the antisense drug she and her husband invent. She has nothing to lose and hopefully everything to gain.