October 3, 2017 – A Massachusetts Institute of Technology spinoff hopes to create battery storage technology capable of replacing fossil fuel-based power as the primary source of baseload electricity delivery across the grid. By doing this 50% of present-level carbon emissions generated by U.S. power generating utilities can disappear.
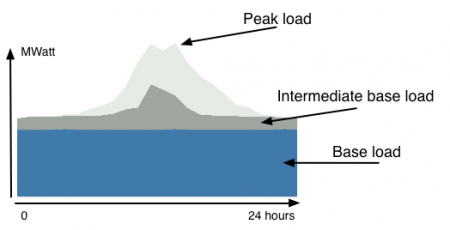
Ted Wiley, Harvard Business School, and a founding member of the company, Baseload Renewables, states, “You can currently put batteries next to solar and wind power and create an output for four hours, but we want to develop a battery to shape renewables into a 24-hour block.”
The initial goal has focused on decreasing the cost of energy storage by a factor of five. To do this the company is looking at sulfur which costs less than any other element when used to store electrons. Abundant and ten times more energy dense than lithium-ion, developing sulfur-based battery technology would be a global game changer.
What Baseload Renewables is out to invent is a flow battery using a polysulfide solution containing chains of sulfur atoms. In the MIT patent application that inspired the spinoff, the technology is described as an air-breathing aqueous sulfur rechargeable system.
Flow batteries use two tanks filled with liquids that are electroactive. One is designated an anolyte, the other a catholyte. The two are then pumped into a cell containing a permeable membrane which allows positive and negatively charged atoms to pass through.
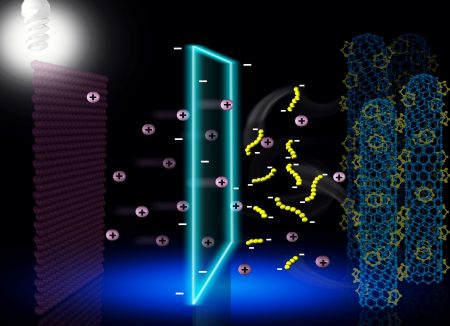
In the patented technology the catholyte contains metal salt dissolved in water. Oxygen gets generated in the catholyte during charging and is consumed when discharging. Getting the chemistry right will make this battery capable of:
- feeding electricity to the grid for more than 24 hours without recharging
- lasting through many tens of thousands discharge and recharge cycles without losing capacity
- durability for 20 or more years
Using waste sulfur, a byproduct of current fossil fuel production solves another environmental problem. There aren’t enough industrial uses for the current volume of the stuff from refining fossil fuels and synthetic crude. As a result, it gets stored in vast yellow mounds adjacent to these facilities.
Just how much sulfur comes from petrochemical refining? One example: for every 72 liters (19 gallons) of gasoline produced, a quarter kilogram (half a pound) of sulfur gets left behind.
If Baseload Renewables perfects the battery in the next two to five years, therefore, the company has the opportunity to solve two pollution problems: removing carbon in the air by replacing fossil-fuel generated baseload power and using up the surplus sulfur that today has no industrial purpose.







