Battery technology is advancing at a pretty good clip these days as the world warms not just in temperature but in embracing the transition to a low-carbon future.
The most recent breakthroughs largely focus on either perfecting lithium-based power storage or moving to other materials that are less fire-prone, charge faster, last longer, and can be mass-produced cheaply.
Among the latest milestones are:
- Advancements in solid-state and semi-solid-state batteries with Toyota recently announcing its strategy to build EVs with operational ranges of approximately 1,000 kilometres (621 miles) using solid-state batteries by 2026 and hybrid models with smaller solid-state battery packs by 2025.
- Recent announcements by QuantumScape, a company that has developed a solid-state battery capable of an 80% charge in 15 minutes.
- Sodium-ion (Na-ion)batteries are becoming an alternative to lithium-ion with several Chinese EV builders launching models and Natron Energy beginning commercial production for heavy-duty vehicles running on sodium-based, not lithium battery packs.
- Magnesium-based batteries to replace lithium-ion for small devices have been invented by researchers at the University of Waterloo.
- Stellantis N.V. and Zeta Energy Corp. are jointly developing Lithium-sulphur (Li-S) batteries for EV models providing higher energy densities and faster charging speeds than existing Li-ion battery packs.
Add to these the latest announcements about a battery that is made from organic materials that store protons and a battery that would use the carbon dioxide (CO2) abundance in the Martian atmosphere for a battery designed to have potential applications for future missions on Mars.
Proton Battery from Down Under
The University of New South Wales (UNSW) in Sydney, Australia recently announced that scientists in its chemistry department have created a rechargeable proton battery that does not run on lithium. Its name is the Benzoqauinone Derivative Anode for All-organic Long-cycle Aqueous Proton Battery according to a research paper published last October in Angewandte Chemie, a German chemical society journal.
The organic material used for the battery anode is tetromino-benzoquinone. The battery rather than using lithium uses protons from hydrogen atoms that have shed their electron. Finding the right material has taken time and the UNSW team feels it finally has found it although currently it is not cheap to produce. In addressing the cost of the battery material, Professor Chuan Zhao, seen in the picture at the top of this posting, notes that “because it’s made of abundant light elements, it will be easy and affordable to eventually scale up.”
The battery’s electrodes are both made from tetromino-benzoquinone. The electrodes are separated by a water solution. Compared to Li-ion which uses lithium salt and an inflammable solvent, the proton battery is safe. No exploding Teslas and laptop computers will happen using proton battery technology. Because protons from stripped hydrogen atoms are used, there is no fear of hydrogen explosions. He notes that the growth in hydrogen infrastructure as the gas becomes increasingly used as an alternative to fossil fuels will allow hydrogen protons to become available anywhere for harvest as needed combined with the new proton-storing material discovered by him and his UNSW colleagues.
A Battery for Mars
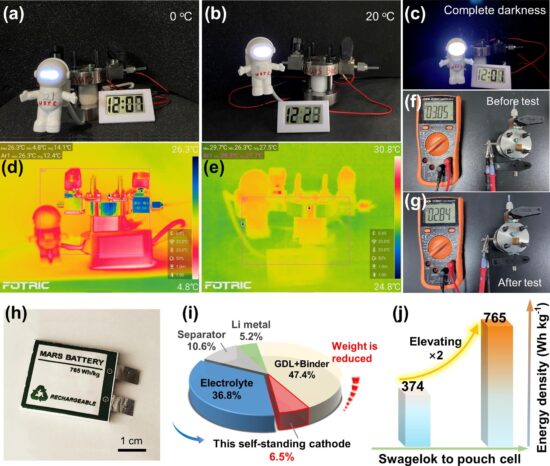
An article recently published in Elsevier’s Science Bulletin describes the invention of a high-energy, high-density battery that could become the go-to technology for providing battery storage to power a future Martian infrastructure.
The current active NASA rovers, Curiosity and Perseverance run on energy packs that use nuclear batteries that generate electricity from heat produced from natural radioactive decay of onboard plutonium-238. Other Martian rovers and the first deployed helicopter used solar energy generated from photovoltaic panels.
For long-term Martian presence, using in-situ materials would be ideal in the creation of an energy source to back up renewable solar. A research team at the University of Science and Technology of China (USTC) decided to try and build a new battery that would use the Martian atmosphere as its fuel source. The result is a Lithium-carbon dioxide (CO2) battery.
The atmosphere of Mars is over 95% CO2 making the material abundant and easy to harvest in making a lithium carbonate battery. The bigger challenge is getting the battery to operate consistently in the low-temperature Martian environment where temperatures can plunge at night to -73 Celsius (-100 Fahrenheit) at the equator, and -143 Celsius (-225 Fahrenheit) at the poles.
The USTC team simulated a narrower temperature range than the most extreme Martian environments. They tested the battery’s operation in temperatures between -20 and -60 Celsius (-4 and -76 Fahrenheit) and were able to produce reliable output running over two months using a design with an integrated electrode and foldable battery as a proof of concept. The battery was able to run several electronic devices (see image below) in these simulated Martian conditions. The research team sees future iterations of their technology as being part of a multi-energy supply system for Martian exploration.