In our last blog we introduced telomeres, the genetic information that slowly vanishes from chromosomes each time cells divide. Researchers who study aging see a correlation between those vanishing telomeres and growing older. But I am getting ahead of myself. Before we can talk about the mechanism of stopping aging we really need to understand aging processes and current theories. Scientists have differing opinions on what causes us to age. One opinion states that our bodies have a biological built-in timeline that switches genes on and off, alters hormones over time and impacts our immune system making us less capable of fighting off disease. The second opinion asserts that we are victims of our environment and the damage it does to us over time. That damage includes genetic mutation in cells, accumulated proteins that impair cell function, and general wear and tear.
Our Biological Limits
In the latter part of the 18th century the average human lived 24 years. By the end of the 19th the average lifespan had doubled. In the second decade of the 21st century we are approaching a doubling again. Is there a biological limit?
We are the sum of our genetics. How long your parents lived may indicate how long you will live. But then again it may not. We know that altering genes can alter the lifespan of animals and plants we study in laboratories. We have doubled the lifespan of mice by splicing genetic material into their chromosomes.

In an earlier blog we described the structure of DNA, genes, base pairs and chromosomes. It may be helpful to click on the link provided as a quick refresher before reading further.
We have two DNA repositories in every cell in our body (blood cells not included). That DNA is found in the nucleus and mitochondria within the cell. The DNA organizes itself as chromosomes. When cells replicate the chromosomes divide and copy themselves. This is called mitosis.
Telomeres are repeating DNA base pair sequences that sit at the end of each chromosome. They act as buffers to ensure that DNA replication during mitosis remains accurate. A ribonucleic protein enzyme, telomerase, maintains the telomeres. As cells divide some telomere information does not replicate, usually between 25 and 200 base pairs. The average telomere can be as long as 15,000 base pairs so what is lost is not significant until the cells divide many times. The accumulation of lost base pairs starts adding up.
Why do we lose telomeres? This may be a reflection of our natural aging process, the unwinding of our biological clock so to speak. Or telomeres may shorten because of external forces such as exposure to toxins, disease or injury. Ultimately when chromosomes no longer have a telomere buffer cells they no longer can divide and we call this cellular state senescence. The Hayflick limit, named after the scientist (see the image below) who, in 1961, first discovered this phenomenon, is the natural limit of a cell’s life after multiple replication.

Do all cells have a Hayflick limit? Apparently not as scientists have observed in studying cancer. Tumor cells do not suffer from DNA strand shortening. They can infinitely replicate because in cancer cells telomerase remains active restoring telomere length. Other cells that don’t exhibit the shortening of telomeres include sperm and egg cells. Hormones may impact telomerase activity and telomere lengths. It is believed that estrogen plays a role and may explain why women live on average longer than men.
If we were to alter the behaviour of our normal cells by stimulating telomerase could we reverse the aging process, ending senescence? This is possible. Use of telomerase in laboratory settings has shown that it can confer “immortality” on several types of human cells. That same capability makes telomerase one of the key factors in cancerous tumor cell growth and is leading to research into telomerase inhibitors that would transform cancer cells by starving them of the protein and putting them into senescence.
Telomeres alone do not extend life. If they were the sole means by which we could stop aging we have the technology to produce on mass altered cells containing high levels of telomerase and bank these for use to cure incalculable diseases. Right now scientists believe that stopping telomere shortening may add 10 to 30 years to the average life span. That would mean a child born today could expect to easily achieve an average lifespan of a century.
Other Factors to Consider that Impact Aging
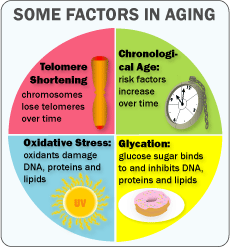
If you are over 60, which I am, your risk dying doubles every 8 years. Research shows that shortening of telomeres only accounts for 4% of the difference. Chronological age and gender account for an additional 33%. The remaining 63% can be attributed to:
- Oxidation
- Glycation
- Inflammation
- Stress
- Immune Response
Oxidation sounds like a strange contributor to aging. After all, we need oxygen to breathe and a byproduct of this basic life function is oxidants. Oxidants result from oxygen combining with sugar to produce energy and byproducts called free radicals. Not all free radicals are internally produced. They can also come from infections, inflammation, alcohol, smoking, excessive sun exposure, radiation from x-rays and environmental toxins. Free radicals can have a negative impact on individual cells, proteins and fatty tissue. Free radicals over time can build up in the body and are associated with aging. In a recent study the lifespan of worms was increased by 44% by neutralizing oxidants.
Glycation involves excess glucose binding with our DNA, proteins and fats. Excess glucose begins to interfere with normal body tissue functions. The older we get the more the glucose creates health problems contributing to aging let alone body mass. Research shows that restricting calorie intake and selecting foods low in sugar leads to reduced age-related disease and extended lifespans in mice to monkeys.
Inflammation is the body’s natural response against infections and injuries. It also contributes to tissue injury and ultimately to aging. Chronic or persistent inflammation without significant infection is evidence of an immune system that no longer recognizes host body tissue as its own. As we age autoimmune conditions including chronic inflammation become more prevalent. Chronic inflammation destroys normal cells and contributes to the aging of the cardiovascular and nervous system. Inflammation contributes to age-related neurodegenerative diseases, such as Alzheimer’s and Parkinson’s.
Stress harms DNA and speeds the aging process. A study in 2004 showed that psychological stress shortens telomeres in immune cells. Evidence shows that stress, the endocrine system response and the occurrence of disease define age more than chronological aging. Certain diseases occur when anabolic hormone levels start to decline and catabolic hormones start to increase. The latter, such as Cortisol, can contribute to the breakdown of body tissue. As proof of just how much stress contributes to aging and premature death, interviewers who spoke with centenarians found that they exhibited healthy coping strategies in dealing with illness describing their behaviours as accepting, non worrying and taking life one day at a time.
Immune Response refers to the specialized cells generated by our bodies to fight off disease. Researchers in Israel at the Technion studied the natural decline in the immune system as we age. This inability to fight off diseases when we are older is one of the reasons for the statistics about the risks to humans when they reach 60 and over. By suppressing B-lymphocyte immune cells in aging mice and using a drug commonly used to treat rheumatoid arthritis, the mice were able to manufacture healthy replacements using bone marrow. Clinical trials have begun in human populations suffering from B-cell lymphoma.
What are the prospects for human immortality?
The SENS Foundation is dedicated to re-engineering our bodies to end aging through rejuvenation biotechnology. These biotechnologies cover major research areas including cell loss, tissue atrophy, nuclear and mitochondrial mutations, immunotherapy, and targeted ablation. The goals are:
- Apply enzymes to lysosomes in cells to destroy the junk that accumulates in them leading to neurodegenerative diseases like Parkinson’s, Alzheimer’s and macular degeneration
- Reduce mutations in the mitochondria of non-dividing cells such as neurons and muscle fibres through applied gene therapy
- Eliminate the extracellular junk that makes artery walls become rigid leading to high blood pressure, or causes amyloidoses in Alzheimer’s sufferers using repair proteins or vaccines to stimulate the immune system
- Remove senescent cells, immunosenescent cells, (white blood cells that no longer work) and visceral fat cells (the fat around our internal abdominal organs that contributes to adult-onset diabetes) cells that accumulate in the body during aging
- Replace lost cells in vital tissues such as brain, heart and skeletal muscles using cell therapy
- Make cancer mutations harmless by interfering with the natural machinery for renewing telomeres
- Develop ways to introduce new ribonucleic proteins into the body and remove those present through transplantation, cell therapy, somatic gene and protein therapy and germ line gene therapy.
If we eliminate all of these physical processes inherent in aging and develop appropriate delivery systems for restoration and repair some scientists project that we can live 1,000 years. Will someone born in the 21st century be the first millenarian?