Of all the topics I have written about to-date, this one strikes closest to home. My daughter was born with congenital heart disease 27 years ago. At the time the odds in favour of her survival to adulthood were low but she made it. Today, children born with her form of congenital heart disease are far more likely to live a full life because of medical discoveries, development of new devices, better medical protocols, and a better understanding of the science of human physiology.
A Quick Primer on Heart Disease
Humans get two types of heart disease: congenital (that is born with) and acquired (disease that develops over time). Congenital tends to be structural. It can express itself in abnormal anatomy or physiology. Acquired begins with normal anatomy but physiology changes as the heart becomes diseased. Both can lead to heart failure which you can live with for a very long time or heart attacks which can lead to death.
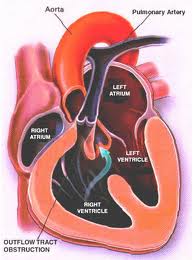
Structural defects to the heart and blood vessels in congenital heart disease can be repaired surgically, missing valves implanted and blood flow restructured using human grafts and artificial materials. Drugs can help regulate heart rhythm or improve the pumping efficiency. Pacemakers can help overcome structural problems that impact the electrical system that regulates heart rhythm.
Acquired, which represents the bulk of what we normally think of as heart disease, can also benefit from surgical intervention, drugs, pacemakers and other devices.
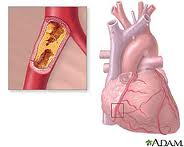
What causes heart disease? Lifestyle and inherited genes are the principal cause with 30 to 60% of the risk associated with the latter when it comes to acquired, rather than the former. Recent international studies identified 17 new genetic variants linked to increased heart disease risk. Five of these were associated with genetic regions that govern lipid metabolism processes, genetic controllers that impact the build up of fatty deposits in arteries. Ten others were in genes involved with other diseases and traits such as autoimmune diseases like Celiac and Type 1 Diabetes, cerebral and abdominal aneurysms, and lung cancer. What this means is there is no single genetic “smoking gun” that defines whether a person is likely to get heart disease in his or her lifetime. Instead we have a complex of genetic markers.
New Therapies for Repairing Bum Hearts and Blood Vessels
Stem Cells
In past blogs we have talked about stem cell therapy. Because stem cells have the potential to develop into a range of tissue types we can use them to regenerate and replace diseased tissue.
Cellular Dynamics International (CDI), is an American company located in Madison, Wisconsin, that is experimenting with the reprogramming of adult somatic cells to make them revert to a pluripotent state so that they can grow into any cell type needed for doing repairs to the body. These are induced pluripotent stem cells or iPS. Since 2008 CDI has used iPS cells to manufacture cardiomyocytes (heart muscle cells), freezing and sending them to pharmaceutical companies doing research on new drugs to treat heart disease.
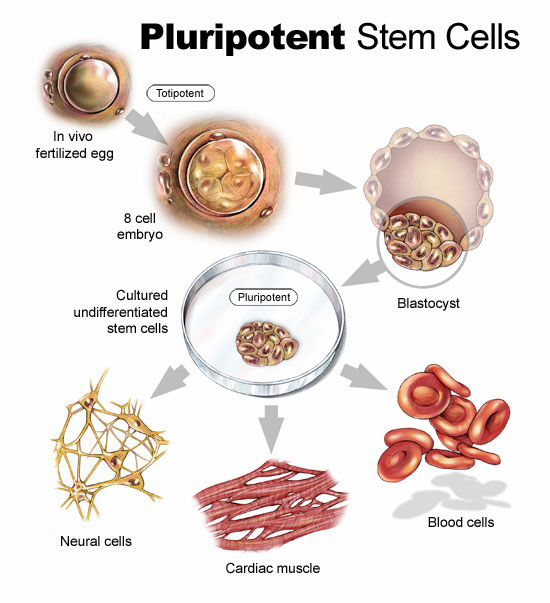
But iPS cells have a brighter future if we can work out some of the bugs. Those bugs include faint traces of chemical residue from the parent cells used to create the reverted cells. Those residues can lead to mutations and as we have already written in previous blogs, mutations can lead to runaway cancer cell proliferation.
You can create iPS cells from almost any human tissue source. If a patient with a damaged heart provides a tissue sample, the cells from the sample can be induced to revert to iPS cells. They then can be transplanted back into the patient at the location requiring treatment. iPS cells from the donor are not rejected because they are from the donor who is also the recipient. The iPS cells in their reverted state can take on the characteristics of those cells that are in proximity to them and start to divide. iPS cells in the heart can generate heart muscle. iPS cells in the spine can generate nerve cells. CDI charges pharmaceutical companies $1,500 for 1.5 million iPS cells. That’s not cheap but the potential for iPS as an alternative therapy to transplants makes this a desirable area of ongoing research.
In research being conducted in Bristol, England and Glasgow, Scotland, Doctor Costanza Emanueli, along with a team of scientists are studying a variety of approaches to treating heart attack victims including using embryonic stem cells to create blood vessel cells to inject into patients. The method of delivery is a skin patch.
Gene Therapy
Researchers are looking at ways to tweak DNA to repair injured hearts. This involves manipulating a variety of genes that impact muscle and blood vessel growth.
Dr. Emanueli’s team, mentioned in the previous section, is studying boosting levels of nerve growth factor (NGF) to improve heart attack survival rates. In studies with mice the death rate from heart attacks was reduced by half using NGF. The gene responsible for NGF was attached to a specially engineered virus and delivered into the hearts of mice. Mice that received the NGF gene showed much improved circulation and heart function. In previous studies NGF was known to regrow nerves but in this case it encouraged new blood vessels to grow inside the injured heart muscle.
In another study conducted at Harvard’s Beth Israel Deaconess Medical Center, the gene C/EPB-beta spurred the growth of cardiomyocytes. This study showed that a genetic trigger responding to physical exercise turned on a molecular pathway to get cells to start dividing. For a very long time cardiologists could not find evidence of cell division in adult heart cells. Hearts under stress or subject to exercise would swell but cell proliferation in these enlarged hearts was poorly understood.
Heart muscle enlargement, called hypertrophy, can be associated with disease or with what is referred to as “athlete’s heart.” In the study cardiomyocyte growth was attributed to molecular events rather than factors such as high blood pressure.
Reprogramming Heart Cells After a Heart Attack
Heart attacks in the past have caused irreparable damage to heart muscles. What causes them? The usual suspects are accumulations of deposits on the walls of arteries that supply the heart with blood. When an artery is partly to fully blocked oxygen and nutrients are cut off to the area next to the blockage. This kills the muscle near the blockage affecting the heart’s ability to beat. But what if the section of damaged heart muscle can regrow?
Professor Paul Riley, of Oxford University’s Department of Physiology, Anatomy and Genetics, and his team of researchers are looking at a layer of cells known as the epicardium. The epicardium is the inner wall of the sac of tissue known as the pericardium that surrounds the heart. The embryonic epicardium contains many types of cells used by the heart and circulatory system including blood vessels and heart muscle. But after birth the epicardium gets quiet and stops generating these tissues. Dr. Riley and his team looked at ways to switch the epicardium cell generation back on. In mice studies they achieved just that by administering doses of Thymosin Beta 4, a natural occurring protein, to stimulate the embryonic genes in the epicardium to create cardiomyocytes. The researchers found that administering Thymosin Beta 4 before a heart attack proved to stimulate faster regrowth of heart muscle tissue.
The team continues to hunt for other naturally occurring molecules that can stimulate heart muscle and blood vessel growth. Along with drug and interventional therapies to clear arterial blockages, administering these proteins pre-emptively could create a bank of healthy new heart muscle to speed recovery should an attack occur.








Your articles continue to fascinate Mr. Rosen. Thanks for making the complex more simple.