From theoretical to deliverable, gene therapy begins its leap to commercial use next year starting with a treatment for lipoprotein lipase deficiency (LPLD).
What is LPLD? Lipoprotein lipase (LPL) is a protein that metabolizes fats in the form of triglycerides and cholesterol. When we eat fats they begin to be broken down in our small intestine. The fatty fragments then get absorbed through the intestine wall into nearby blood vessels and reassemble as lipoproteins. The largest of these lipoproteins is chylomicron and it plays an important role in the transporting of triglycerides to all parts of the body to produce and build energy stores. When a person suffers from LPLD the lack of the protein causes the body to produce many more chylomicrons, clogging up the blood.
People with LPLD have an altered gene responsible for the deficiency. Their blood has high levels of fat. They suffer from abdominal pain. And they are at risk to develop pancreatitis. LPLD affects one in a million but has higher concentrations in specific geographic areas such as parts of Canada.
Before the development of a gene therapy the standard treatment for LPLD focused on reducing blood fat levels through diet or drug therapy. Those who develop pancreatitis receive pain relief medication. There is no cure that is until now.
Enter uniQure, a Dutch-based pharmaceutical company that is doing extensive research in the field of gene therapy and is the first to market with a product, Glybera, aimed at treating LPLD. In the last month Glybera received approval from both the FDA and the European Commission and will be available for patients with LPLD in 2013. Glybera is just one of many new gene therapy products uniQure has under development. These include therapies for:
- the blood clotting disorder hemophilia B,
- the metabolic disorder acute intermittent porphyria,
- the nervous system disorder Parkinson’s,
- and Sanfilippo B, an enzyme disorder.
Glybera is not the first gene therapy treatment on the market. Back in 2003, China approved a gene therapy product, Gendicine, to treat head and neck squamous cell carcinoma, a cancer responsible for 10% of new cancer diagnoses each year in China. Gendicine works to restore the normal function of the p53 oncogene. Gendicine has been used in trials to treat bladder and liver cancer, and mesothelioma, caused by exposure to asbestos.
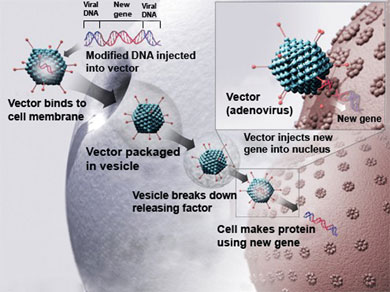
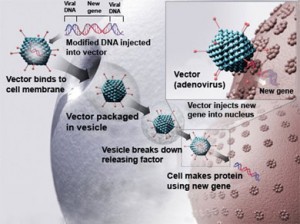
Both drugs use a modified virus as the delivery agent. The virus gets injected into body tissue where it infects the surrounding cells with a healthy gene that then gets copied to replace the cells with the genetic defect.
Current gene therapy research is focused where known genetic defects are linked to diseases. These include Down’s syndrome, cystic fibrosis, Duchenne muscular dystrophy, child blindness, hearing disorders, many types of hemophilia, a wide range of cancers, and a number of cardiovascular disorders. A list of clinical trials can be found at the Gene Therapy Network.
Gene therapy doesn’t come cheap. Gendicine treatment can cost up to $100,000. Glybera will cost $1.6 million per dose.