My daughter was born with a congenital heart-lung defect. One of the options my wife and I faced was to permit the doctors to perform a heart-double lung transplant. Fortunately we didn’t choose to exercise that option and our daughter survived many heart surgeries and multiple bouts of pneumonia with her own organs intact. Today she is 28 and although she will have heart operations in her future, even in their diminished state, her lungs will not have to be replaced. For my wife and I this is a great relief because of all the organs that get transplanted, the lungs are the toughest to get right.
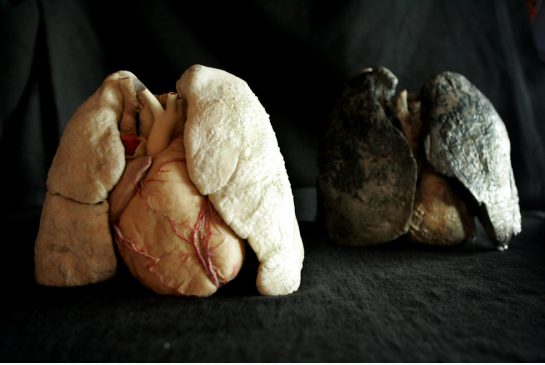
Today 80% of donor lungs get rejected by transplant teams. The image on the left in the picture below is a healthy harvested set of lungs and heart. The one on the right is an example of a harvested heart-lung rejected by a surgical transplant team.
There are all kinds of reasons for determining unsuitability. Donor history is one. Did the donor smoke? Yes…..rejected. Did the donor suffer from chronic respiratory infections? Yes….rejected. Did the donor’s lung x-rays show unusual patterns in the airways and blood vessels? Yes…..rejected.
In addition to history and x-ray information, the fragility of lungs makes them a transplant transportation nightmare. Lungs have to keep on breathing so that blood vessels and aerioles survive undamaged. And then you have the problem of getting a good physical match. This is not just matching blood types to ensure a degree of compatibility. This is truly a physical issue. Lungs sit inside the rib cage and chest wall and donor and recipient have to have similar physical dimensions otherwise the lungs cannot be accommodated. You just can’t cut a bit of lung off if the fit isn’t quite right. Getting the fit right is even more complicated when you realize that lungs change shape when you breathe in and out.
And then there is a complication that often happens after a lung transplant called primary lung graft dysfunction or PGD. This usually occurs within the first 72 hours after a successful transplant and features severe swelling with not enough oxygen getting to the new lungs. PGD occurs in 10 to 25% of all lung transplant surgeries.
Two new technologies, however, are about to alter lung transplant statistics forever.
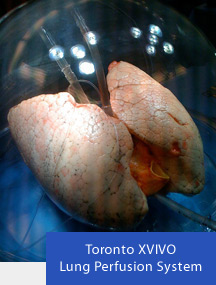
One is a Swedish invention that has been undergoing clinical trials in Canada and the United States. The company, XVIVO Perfusion Inc., produces a cell-free solution that is pumped into lungs to protect them after harvesting. Called Steen Solution after its surgeon inventor, the liquid keeps the lungs clean, reduces the buildup of fluids and prevents clot formation through a process called ex vivo lung perfusion. Steen Solution circulates through the harvested lungs for several hours. If the lungs continue to exhibit normal function they are deemed suitable for transplant.
In the Canadian study conducted at Toronto Hospital (see image below) and other transplant centres across the country, 50 of 58 lungs (86%) first considered to be risky were deemed suitable for transplant. Using the technology has seen more than a doubling in the lung transplant pool to 45% of all donated organs. In the American study at University of Pennsylvania and five other medical centres similar results are being reported.
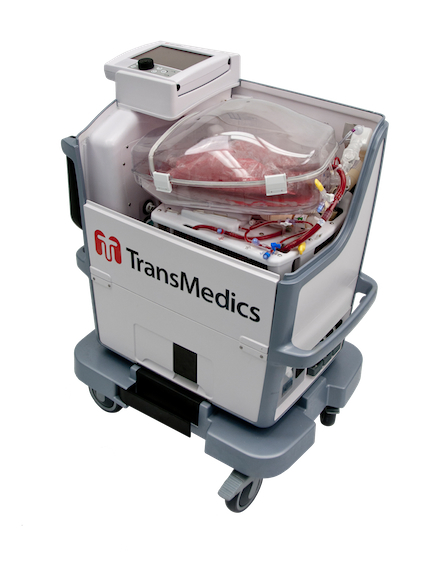
The second technology comes from TransMedics, a company located in Massachusetts. It has developed the OCS Lung, the world’s only portable lung perfusion system. The device oxygenates, warms and monitors lungs during transportation to keep them viable much longer than older methods which involve packing harvested organs in ice. OCS Lung is just starting clinical trials in North America and is already in use at hospitals in Europe and Australia.