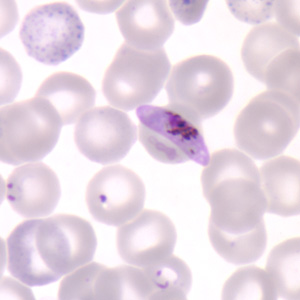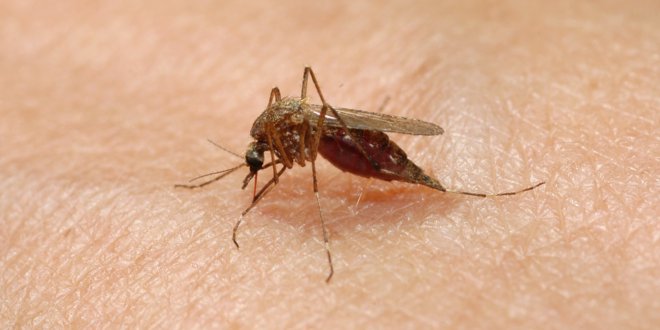
Clinical trials have started for a malaria vaccine dubbed the PfSPZ Vaccine (for Plasmodium falciparum, the strain of malaria it treats) and the initial results are very promising generating an immune system response in a high percentage of adult volunteers enrolled in the study.
The initial trial in Bethesda, Maryland was done at the U.S. National Institute of Health Clinical Center and involved 57 healthy adults between ages 18 and 45 who had never had malaria. Of the 57, 40 received vaccinations, some with higher doses and some lower. There were no observed adverse side effects. To evaluate the effectiveness for controlling malaria both vaccinated and unvaccinated were exposed to a controlled human malaria infection. The participants were monitored to see what level of vaccine dosage proved effective. The results of 15 who received the highest dosage of vaccine only 3 became infected. Almost all who received low doses of the vaccine became infected as did almost all participants who received no vaccine. All who were infected received anti-malarial therapy and were cured.
Malaria is a scourge in Africa, India, Central and South America and the South Pacific. It kills between 490,000 and 836,000 every year. In 2010 the World Health Organization recorded 219 million cases of the disease. The direct costs of the disease is estimated to cost $12 billion per year.
The researchers hope to do follow up studies to refine dosages and dose schedules, as well as to develop new vaccines against other Plasmodium strains of the disease. The eventual goal is to create a subcutaneously administered serum. In the clinical trials the doses of PfSPZ were administered intravenously.