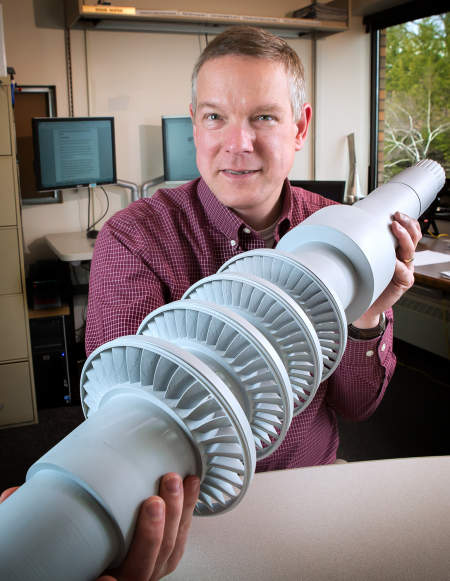
April 13, 2016 – General Electric’s (GE) Global Research arm is testing a turbine (seen in the picture below being held by Doug Hofer, the GE engineer in charge of the project) powered by “supercritical carbon dioxide” or ScCO2. What makes CO2 become supercritical and what exactly does that term mean?
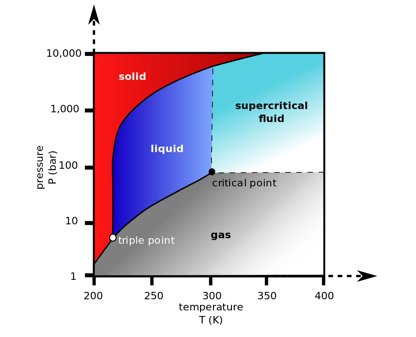
Supercritical is defined as a state above a critical threshold. In reference to a fluid, it is the state when one cannot distinguish between gas or liquid phase for the substance. The graph below shows the critical point when a gas or liquid enters supercriticality. In this state produced by exposure to high pressure and high temperatures supercritical fluids such as ScCO2 display interesting properties. For a demonstration of just what happens watch this YouTube video.
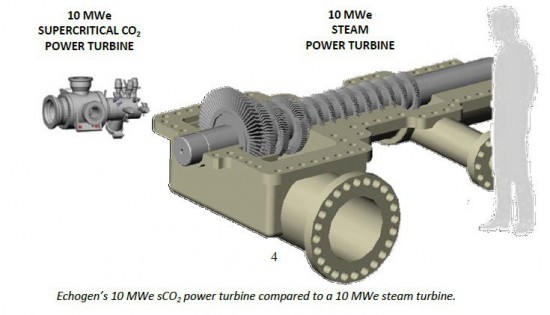
Using ScCO2 in power turbines has been of interest to the energy industry for a number of years. Why? Because as an energy driver ScCO2 remains stable compared to steam. It also packs more punch per square meter because of its small form factor as you can see in the illustration below. In addition to being more stable, ScCO2 turbines produce more energy because of 20% higher efficiency rates than steam.
So why haven’t we seen mass adoption of this technology by power utilities?
The challenge in the past has been to find the right materials capable of withstanding the elevated temperatures and pressures required by ScCO2 turbines. That’s what GE’s engineering team set out to do. And they think they got it right in developing a ScCO2-powered turbine prototype capable of generating 10 Megawatts in a form factor no bigger than a desk. There next step is to scale it to 33 Megawatts.
ScCO2-powered turbines do not require as much energy input to reach supercritical phase and they can be up and running within a minute or two. That’s up to 30 times faster than bringing a steam turbine on line.
ScCO2 has another advantage. It doesn’t corrode or fatigue materials like steam. And the technology is lower maintenance because it has no associated water quality and treatment costs which are necessary for steam-based turbines.
GE believes this technology is a good fit for providing spot-power generation for utilities during peak demand periods. It also sees it as a substitute for storage batteries at solar thermal plants where it can use heat from the molten salt reservoirs to fire up and provide continuous power even when the sun is not shining.
But GE is not alone in using ScCO2-turbine technology. Another company, NET Power has been building a power plant that generates zero emissions employing a similar technology to that of GE.
How does the NET Power technology work?
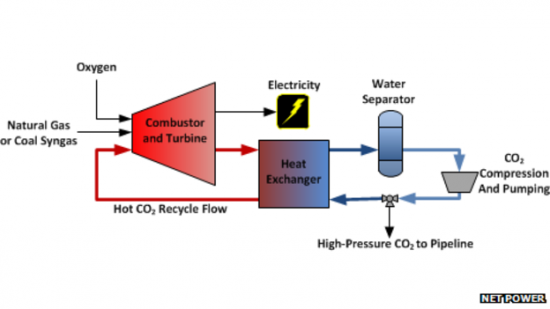
The above diagram illustrates the process. The source of fuel can be coal or natural gas. When burned with pure oxygen produced in an Air Separation Unit it also produces CO2. The latter is subjected to high pressure and heat in an oxy-combustion process. That turns the CO2 into ScCO2 which then powers a turbine.
The byproducts of the energy generation include water and CO2. Both are captured and go through a heat exchanger where the water condenses and is recycled while the CO2 either loops back to the turbine or gets sent through a high pressure CO2 pipeline and pumped underground into oil wells to enhance petroleum recovery.
Like the GE technology, NET Power doesn’t require a large footprint saving significant space compared to traditional coal-fired or natural gas power plants. A 50 Megawatt demonstration power plant, the first of its kind, is expected to be up and running in Texas by the end of 2016.
Imagine the potential this represents for both addressing energy demand while utilizing fossil fuel sources for power. The potential big fly in deploying lots of NET Power technology is the viability of a strategy where sequestration of CO2 is a key component. That may limit site selection because proximity to oil fields or to a stable underground rock formation where the CO2 when pumped underground in a super critical state would bind with the existing minerals. The last thing the industry would want to do is potentially create a CO2 leak to the surface negating the purpose of the sequestration effort.