March 30, 2020 – The traditional protocol and timelines for developing new vaccines is anywhere from 6 to 18 months and sometimes longer. Formulating a vaccine isn’t the only challenge. Clinical trials and peer review results add months to the development process. And then there is the final step, regulatory approval which in the United States comes from the Food and Drug Administration (FDA) and Health Canada in this country.
The influenza vaccine we formulate to deliver to millions of Canadians each fall gets developed in a six-month window and is based on changes to the viruses observed on the opposite side of the equator. So when Australia gets inundated with the flu, the latest version of the viral outbreak is broken down to develop a vaccine countermeasure which gets administered in the Northern Hemisphere before the advent and during flu season. And any changing characteristics experienced this side of the equator influence the flu vaccine subsequently administered Down Under before their season commences.
Other vaccines don’t go through such a quick process as does flu. In the case of COVID-19 (the acronym stands for coronavirus disease 2019) there is no immediate existing viral infection upon which to model a vaccine. The closest coronavirus kin are SARS-COVID-2, an outbreak in China that traveled overseas here to Toronto where it killed many in 2002, and MERS-COVID-12, a Middle-Eastern originating coronavirus that struck in 2012. In both SARS and MERS no specific vaccine has ever been developed which means, we have nothing in our global medicine chest upon which to base a preventive treatment.
Treatment for COVID-19 is approximating that which was put in place for SARS and MERS. The only difference is the overwhelming size of the COVID-19 outbreak compared to the previous coronavirus epidemics. It is the explosive growth in the number of infections that is overwhelming existing medical infrastructure. Hospitals and the worldwide medical community seem to have been caught unaware despite the fact that the initial outbreak in Wuhan, China, was very much in the news as early as December of 2019.
Right now the way organized medicine is fighting COVID-19 is to wait out those experiencing the worst symptoms by putting them on ventilators until the virus runs its course. At the same time, hospitals are turning to a stock of older drugs to try and see if any can ease a patient’s symptoms. Among these are:
- Chloroquine and Hydroxychloroquine – antimalarial drugs that President Trump has hyped as a potential cure.
- Lopinavir and Ritonavir – both were used in SARS and HIV treatment as protease inhibitors, but have shown little effect in dealing with COVID-19.
- Remdesivir – a broad-spectrum antiviral that inhibits the virus’s protein production with several clinical trials currently underway.
- Azithromycin – an antibacterial adjunct therapy that when combined with chloroquine or hydroxychloroquine may have limited application.
- Tocilizumab – an immunomodulating adjunct therapy that shows some promise in patients with severe manifestations of the disease.
Then there are some very recently announced approaches that include:
- COVID-19 convalescent plasma serum from recovered COVID-19 patients containing antibodies to the virus that could accelerate treatment.
- Peptide-based protein compound from MIT that binds to COVID-19 proteins preventing them from taking over the cells inner protein replicating machinery.
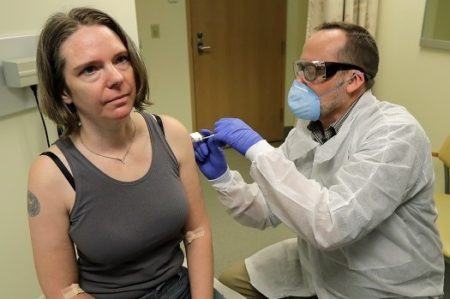
And finally, there is a race to develop the first vaccines from a number of different providers including:
- Arcturus Therapeutics working with Duke-NUS Medical School of Singapore which are in the process of developing a self-replicating RNA-based therapeutic vaccine to protect humans from COVID-19.
- Moderna Therapeutics in Massachusetts, already testing an experimental vaccine called mRNA-1273 for COVID-19 working in conjunction with Kaiser Permanente Washington Health Research Institute in conjunction with the permission of the National Institutes of Health (NIH). The initial study involves 45 human volunteers.
In the title of this article, I suggested that COVID-19 could alter our approach to finding new treatments for novel diseases humanity encounters in the future. It would seem that necessity is driving us in this direction. Why?
Here are the latest coronavirus pandemic statistics as of 3:00 p.m. Eastern Daylight Savings Time, March 30, 2020:
Number of COVID-19 cases as of right now: 770, 165 compared to 86,604 on February 29th, one month ago (889% increase). What will it be a month from now? Over 6.8 million cases.
Number of those who have died: 36,938 compared to 2,977 on February 29th (1241% increase). What will this number be a month from now at this rate of growth? Over 458,000.
With these types of numbers, making haste in the testing of new therapies and trialing vaccine trials makes sense. The old clinical trial review processes may just not cut it in the face of the explosive growth potential of this pandemic.