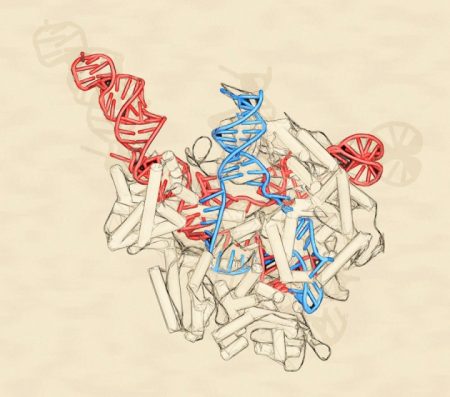
October 15, 2017 – The DNA editing tool known as CRISPR is about to tackle the creator of aberrant proteins that lead to Huntington’s Disease and Amyotrophic Lateral Sclerosis (ALS), also known as Lou Gehrig’s Disease. CRISPR, an acronym for a tool that bears this long title, Clustered Regularly Interspaced Short Palindromic Repeats, has been used to cut out defective genes in DNA and replace them with healthy ones. CRISPR does this by using an editing tool, a protein designated as Cas9 to execute the cut and replace procedure. But CRISPR until now hasn’t tackled RNA, the molecule that transfers and decodes genetic information found within cells.
RNA, or ribonucleic acid, is DNA’s right-hand man. It is referred to as the messenger turning genetic instructions into proteins responsible for healthy operations within cells. When defective the proteins it constructs lead to disease.
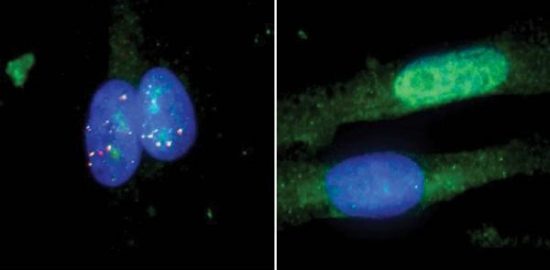
Researchers at the University of California, San Diego (UCSD), decided to use CRISPR and a modified Cas9 companion to target RNA rather than DNA. The modified Cas9 binds to aberrant RNA and destroys it. In a paper published in August of this year in the scientific journal, Cell, entitled “Elimination of Toxic Microsatellite Repeat Expansion RNA by RNA-Targeting Cas9,” UCSD researchers describe laboratory tests in which 95% of aberrant RNA from cells taken from Huntington’s Disease, and ALS patients were destroyed using CRISPR. The tool was tested as well on a form of inherited muscular dystrophy, myotonic dystrophy. Again it successfully eliminated 95% of the RNA responsible for creating defective proteins.
UCSD researchers describe laboratory tests in which 95% of aberrant RNA from cells taken from Huntington’s Disease, and ALS patients were destroyed using CRISPR. The tool was tested as well on a form of inherited muscular dystrophy, myotonic dystrophy. Again it successfully eliminated 95% of the RNA responsible for creating defective proteins.
The effects of the treatment, however, are temporary with the RNA regenerating within a few days. This is both good and bad. It means using this technology could help tackle infectious diseases with a known RNA origin. But for Huntington’s and ALS a longer term solution is needed. Even here the researchers believe they can use the technology in a lifetime treatment protocol by turning the tool into a daily capsule to keep aberrant RNA in check.
This is far removed from CRISPR/Cas9’s original purpose, but nevertheless, it illustrates the promise of this tool that is, without a doubt, revolutionizing medicine.
A new biotech startup, Locana, has been created to move the technology from the laboratory to commercial application. We should be hearing much more from Locana in the next few years.