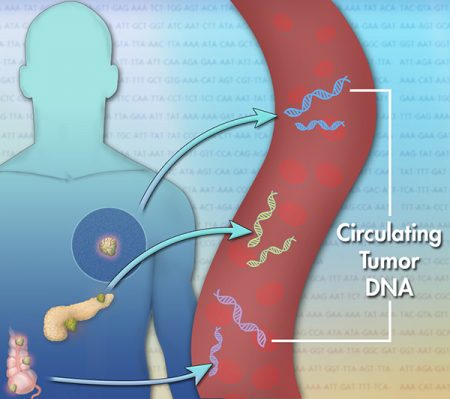
January 10, 2017 – On this date last year, Illumina, a San Diego-based company that makes the machines scientists and doctors use to sequence DNA, announced it was forming a spinoff named Grail. Grail would be developing a cancer detection test based on blood-work DNA analysis. Grail raised more than $100 million U.S. to date to help it in its pursuit. And now, based on last week’s announcement, plans to raise an additional $1 billion. There is lots of interest from the investor community.
The blood searches for snippets of DNA shed by cancerous tumors into the bloodstream. The technique has successfully been used on expecting mothers to detect Fetal Down Syndrome. It has also detected liver and naso-pharyngeal cancers. Jeff Huber, the CEO of the company, has a very personal stake in making liquid biopsies a success. He lost his wife to colorectal cancer.
What makes detecting cancer from a blood sample difficult is that cancer tends to mutate rapidly. To be successful the test must compare blood samples taken from a large database where DNA from tumors have been detected. That’s what much of the money raised will be spent on. Large-scale trials, involving hundreds of thousands of patients, will serve two purposes. First it will create the cancer DNA database and second, it will demonstrate the capability of liquid biopsies as a non-invasive alternative to traditional cancer detection technologies.
Besides the benefit of being less invasive as a test, a liquid biopsy is something that can be run annually for patients with family histories of cancer. Because cancers detected at late stage are almost always non-treatable, liquid biopsies could help catch cancer at its earliest meaning earlier treatment, dramatic drops in cancer deaths and higher rates of remission and cure. Another benefit of a liquid biopsy is it can be used for selecting appropriate drug therapy to treat a cancer based on the mutation detected in the patient’s DNA. A few drops of blood extracted sure beats having a tissue biopsy taken from a tumor.
Grail is not alone in pursuing development of a liquid biopsy test. Research is ongoing at numerous hospitals including the Dana-Farber Cancer Institute, University of California Davis Comprehensive Cancer Center, Johns Hopkins University, Memorial Sloan Kettering Cancer Center, and University of Texas M. D. Anderson Cancer Center. In 2015, the journal The Lancet Oncology, published a paper describing results comparing liquid and tissue biopsies in detecting colorectal cancer. And last June the U.S. Food and Drug Administration approved the liquid biopsy cobas EGFR Mutation Test for detecting metastatic non-small cell lung cancer.
A personal note, I have a family history of colon cancer and have periodic colonoscopies. If you have had to endure this particularly screening test than you have no idea how appreciative you will be if a blood biopsy replaces it.