August 9, 2014 – A suspected Ebola patient has appeared thousands of kilometers away from West Africa here in the Greater Toronto Area. The patient was admitted to a hospital in the last two days complaining of symptoms equated with early stages of Ebola. The person had recently visited West Africa and may have been exposed to someone infected that was traveling as well. The problem with Ebola at the early stage is the symptoms are flu-like and also can be confused with the onset of malaria. Doctors will know in the next few days if they are dealing with Canada’s first Ebola case related to the latest African outbreak. But they are proceeding as if it were Ebola.
Canadian researchers seem to be at the forefront of trying to find a cure. I wrote about ZMapp in a previous posting, an experimental drug developed at Canada’s Public Health Agency in Winnipeg, Manitoba. The two Americans who contracted Ebola while working with patients in Africa are improving in their fight against the disease. It may be because of the benefits of the drug, or because of the level of care provided, or the timing, or blind luck. We don’t know.
But now another Canadian developed drug produced by Tekmira Pharmaceuticals of Burnaby, British Columbia, is in clinical trial. The U.S. Food and Drug Administration (FDA) had put a hold on the trial to assess side affects, but on August 7 announced a modification to its policy. It has now deemed the trial to be on a “partial clinical hold.” This means the drug can be released for use to help those individuals currently with Ebola as part of larger study. The original clinical trial involved two control groups. One was receiving ascending doses of the drug while the other received a placebo. The goal was to assess drug tolerance and potential side affects.
TKM-Ebola is described as an anti-Ebola RNAi therapeutic drug. What is RNAi? It stands for RNA interference. In previous posts on computational biology I have described the relationship between RNA and DNA. Today our understanding of that relationship is at the heart of a medical revolution and we made the discovery when an agricultural scientist tried to insert a gene in a petunia to make it turn purple. That purple petunia experiment ended up identifying RNAi, a component within our cells that recognizes something bad is happening with normal cellular processes and automatically shuts down the process. In discovering this natural ability within cells researchers have found a tool to destroy viruses which is leading to a whole new range of medical treatments for diseases. Our understanding of RNAi may help us end the scourge of AIDS, and stop diseases like Huntington’s. Tekmira is one of a number of companies in the forefront of this research.
In its work with non-human primates and the administration of TKM-Ebola, the company has produced a drug that provides 100% protection from the Ebola strain found in Zaire outbreaks. And although not all drugs provide equal results when moved from laboratory animals to humans, the fear of a widening Ebola epidemic appears to be shaking the principles of FDA standard protocol.
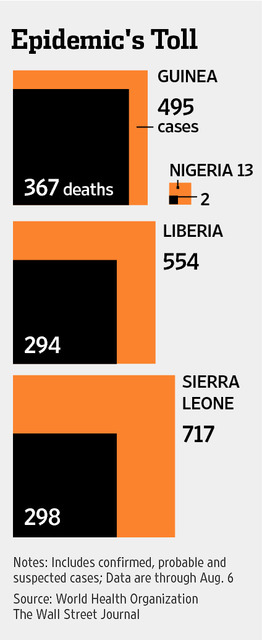
The current Ebola outbreak is the most extensive seen to date. The fact that is has been transmitted through air travel heightens the urgency of the situation. It appears that this is the motive behind the FDA creating an exception to its normally rigorous protocols.
TKM-Ebola is being developed under a $140 million US contract with the U.S. Department of Defense. Before this latest announcement the FDA had fast tracked the drug. Now the FDA has introduced a new protocol which tells you just how seriously it and the World Health Organization consider the current Ebola West African outbreak.