Hydrogen can be found everywhere on Earth. Along with oxygen it forms water. But getting the hydrogen out of water and distributing it for use as fuel has remained a challenge. Hydrogen easily combusts. It is difficult to transport because you need a lot of it to get the equivalent energy you get from other fuels. And because you need a lot we compress it. To turn it into a liquid like gasoline we have had to cool it and then store it in pressurized tanks.
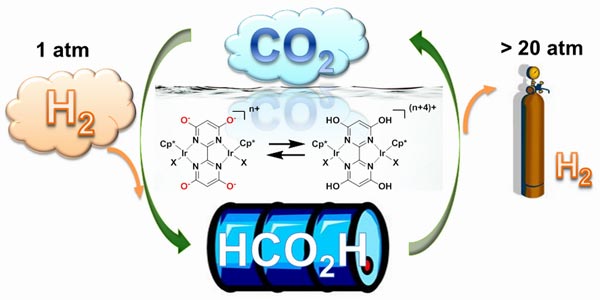
Well according to research by a team led by Jonathan Hull, a chemist at Brookhaven National Laboratory in New York, by combining hydrogen with a water-soluble molecule containing iridium and baking soda, they converted the gas to a form storable in a liquid state at low pressure in ambient temperatures. To reverse the process the researchers added an acid to the solution releasing the stored hydrogen.

The process makes it possible to transport hydrogen easily without the need for high pressure and low temperature storage. For hydrogen fuel cell manufacturers this represents an enormous breakthrough.
The research at Brookhaven was done in collaboration with Yuichiro Himeda of the National Institute of Advanced Industrial Science and Technology in Japan.








