Hydrogen is a $100 billion industry. What do we use it for commercially today? It is certainly not a commonly used fuel except in a few places like Iceland. It isn’t easy to produce, in fact, we have to extract it from natural gas. And when we use it, it is not for energy but for manufacturing fertilizer and for refining fossil fuels which we then burn. And finally our current extraction processes make hydrogen very unattractive because we produce large amounts of CO2 as a byproduct and we know that CO2 is the primary source for the recent atmospheric warming on our planet.
Having said that we know that hydrogen burns. We know this because we have seen pictures of the Hindenburg. We know this because in liquid form hydrogen was the fuel fuel for NASA’s Saturn V rocket that took us to the Moon. So producing hydrogen for energy makes sense if we could avoid the CO2 issue and find an affordable way to produce it on mass. That’s why I keep writing about research scientists and their announcements of test results similar to the one in Alberta that I reported on last week and the ones I am writing about today in this posting.
Hydrogen from Methane Using a Liquid Metal Reactor
Karlsruhe Institute of Technology (KIT) is planning to produce hydrogen from methane without CO2 as a byproduct of the process. The researchers in the Karlsruhe Liquid-Metal Laboratory are setting up a liquid-metal bubble column reactor to decompose methane into hydrogen and carbon under high temperature conditions. The ultimate goal is to produce a sustainable level of production of hydrogen from fossil fuel sources without contributing CO2 to the atmosphere. The technology being deployed is a vertical column approximately a half-meter (about 20 inches) in height and a few centimeters in diameter. At that size it is clearly not meant to be a commercial product. The column itself is filled with liquid metal at a temperature of 1,000 Celsius (1,832 Fahrenheit) degrees. The bottom of the column is porous. Methane is introduced through it and as it bubbles up to the top it decomposes into hydrogen and carbon. The researchers will be studying yield rates using this process as well as methods for removing carbon using a range of filtering technologies.
This liquid-metal bubble column reactor is based on previous work done by the Institute for Advanced Sustainability Studies in Potsdam and by the Argonne National Laboratory that have shown the effectiveness of thermal decomposition as a process in gas-phase reactors. The biggest issue in previous experiments has been the tendency for the carbon to accrete to the reactor walls during decomposition. With a new reactor design the bubbles will be dispersed to form a constantly renewing curtain to keep the carbon away from the reactor walls. Only when the bubbles reach the surface of the liquid metal will carbon get released.
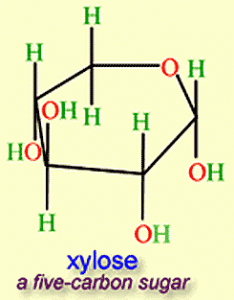
Hydrogen from Plant Sugar
Researchers at Virginia Tech are extracting large volumes of hydrogen from plants with the hope that this discovery will speed up the development of a hydrogen infrastructure to replace fossil fuels. The process for extraction involves xylose, making up as much as 30% of plant cell walls. The technology produces no greenhouse gases and doesn’t require the use of heavy metals.
Following seven years of research in which scientists separated a number of naturally occurring enzymes to create a cocktail not found in nature, they came upon the right combination that when introduced to xylose and polyphosphate released high volumes of hydrogen. The cooker operates in normal atmospheric pressure at temperatures of 50 Celsius (122 Fahrenheit). The process results in energy efficiencies greater than the chemical energy stored in the xylose and polyphosphate. The researchers believe that they can use low-temperature waste heat sources to convert wood sugar into hydrogen at a net energy gain.
Hydrogen from Natural Gas Without CO2
Eindhoven University of Technology is the home of Mohamed Halabi, a researcher who has demonstrated the ability to produce hydrogen from natural gas. That is not unique but what stands out is the lack of CO2 as a byproduct of the process.
The method for hydrogen extraction involves a packed bed reactor that uses a rhodium-based active catalyst and a hydrotalcite-based sorbent, the latter a naturally occurring mineral also known as aluminum magnesium carbonate hydroxide hydrate. So although a tongue twister I think calling hydrotalcite is a lot easier.
How does the hydrogen in this process get produced. The cooker operates at temperatures of 400-500 Celsius (approximately 750 to 950 Fahrenheit) and at 4.5 times normal atmospheric pressure. That releases the hydrogen, captured by the active catalyst, and the CO2, absorbed by the sorbent.
Conclusions
Which of these processes would you place bets on? I’m leaning to producing hydrogen from xylose as the most likely candidate to become commercially viable. The others just sound too high tech and expensive. Send me your pick of the bunch and, if you are an expert in the field, let us know what you think.









By an interesting coincidence, I just got the spring-summer 2013 edition of my `club Toyota` magazine today. On page 36 it states that Toyota will be introducing a hydrogen fuel cell vehicle by 2015. Their expectation is that most of the hydrogen supply will come from natural gas, but the specific technology was not specified.
Toyota isn’t the only one involved in this fuel cell car initiative. I wrote a piece on this some time ago.