January 15, 2017 – When summer produces heat wouldn’t it be great if we could capture it and use it to warm ourselves through the winter. That’s what researchers at EMPA, the Environmental and Marine Project Management Agency of the European Union, have tried to do in developing a storage technology that uses a simple chemical process and not fossil fuels to keep homes warm in wintertime.
Since last fall, EMPA has been operating a system that stores heat using sodium hydroxide (NaOH) and water. The combination produces an exothermic release of heat. The NaOH solution can be stored, shipped in tankers, and reliably be used repeatedly over many years. Translating from a laboratory experiment to single-family homes has required EMPA researchers to scale the application. And they have done it quite successfully.
Heating NaOH solution can be done simply by exposing it to any heat source. In the summer a passive solar collector would do the trick. NaOH can be stored and shipped by tanker. It is chemically corrosive but fortunately off-the-shelf pipes used in industrial and residential plumbing can be used to create what in effect is a closed heating system with a thermal battery.
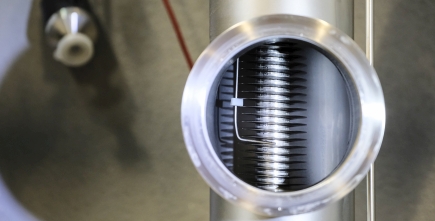
In the picture below you can see what the interior of the heat exchanger looks like. It consists of a pipe in a pipe. The larger pipe contains a 50% solution containing NaOH. The inner spiral pipe contains a 30% solution of NaOH heated by passive solar collectors working much the way rooftop hot water generating systems operate in many parts of the world.
As the more dilute solution passes through the internal spiral pipe it transfers heat to the more dense NaOH solution. That heat energy can then be piped underground to be stored in what is called a geothermal probe, essentially a hot bed of NaOH in solution. A flick of a switch in the system can reverse the process so heat from the geothermal probe can pass up through the solution in the outer pipe and be released. A typical configuration would involve installing a lattice of pipes beneath the floor to serve as the radiant heat source.
The system can be preset to automatically trigger when outside temperatures drop to between 5 and 10 Celsius (41 to 50 Fahrenheit) or lower. The dilute solution in the pipes stays a steady 50 Celsius (122 Fahrenheit) throughout the winter months making for a comfortably warm energy source.
One of the EMPAS researchers, Benjamin Fumey, who co-developed the technology at a laboratory in Switzerland, states, “this method enables solar energy to be stored in the form of chemical energy from the summer until the wintertime……and that’s not all. The stored heat can also be transported elsewhere in the form of concentrated sodium hydroxide solution, which makes it flexible to use.” Fumey (seen in the picture below working on the prototype), and his research associate, Robert Weber, are currently seeking an industrial partner to take the technology out of the lab and into homes.









[…] Uses solutions of sodium hydroxide running in pipes to generate an exothermic chemical reaction converting summer heat into winter warmth. […]