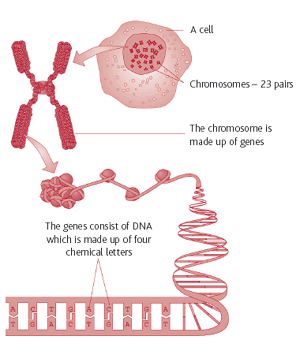
What does final-stage testing mean when talking about gene therapies? It means advanced human trials are on the go and governments shortly will begin approving a number of gene therapy treatments for diseases such as prostate cancer, metastatic melanoma, artery failure in limbs, neurodegenerative diseases, early onset blindness, and a metabolic syndrome called lipoprotein lipase deficiency. The last one I recently wrote about and the developer, UniQure, is already approved in Europe and seeking the green light from the FDA for the U.S.
Currently there are 1,970 gene therapy trials on the go globally. The trial breakdown by gene type include antigen studies, representing 21.2%, cytokine studies, 17.7%, tumor supressors, 8%, suicide studies, 7.9%, receptors, 7.6%, growth factor, 7.3%, replication inhibitors, 4.4%, markers, 2.7% and others and unknown at 15.3%.
Of these the MIT Technology Review this week reports several U.S. groups enrolling patients in late-stage trials. These include trials by:
Advantagene, a bio-pharmaceutical company located just outside of Boston, Massachusetts, that is in Phase 3 trials for ProstAtak, a gene transfer technology delivered by vaccine that jump starts the body’s immune system to detect and destroy prostate cancer cells. The therapy is administered in conjunction with radiation therapy and not only attacks the cancerous tumor but also any recurring or remaining cells that linger after the tumor is destroyed. The Phase 3 trial involves 711 male volunteers with half receiving a placebo. Advantagene is also working on other gene therapies including treatments for malignant and pediatric glioma, pancereatic cancer, mesothelioma, ovarian cancer and esophageal cancer.
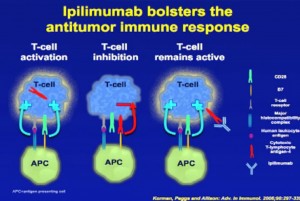
Amgen, a biotechnology company located in Thousands Oaks, California, that is conducting Phase 3 trials for Ipilimumab, a monoclonal antibody that activates the immune system to fight metastatic melanoma, a skin cancer that often spreads to other organs in the body. European clinical trials show survival rates of 18% after five years, and the Dana-Farber Cancer Institute in Boston has presented findings that after 3 years of treatment show overall survival extended to up to ten years. The drug works by targeting the Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) which in cancerous patients is inhibited from recognizing and destroying cancer cells. When administered CTLA-4 is enabled so that it attacks the cancer.
AnGes MG, a bio-pharmaceutical company located in Osaka, Japan, that is developing Hepatocyte Growth Factor (HGF), a blood vessel regenerator to cure hepatic diseases caused by ischemia, diabetes, arteriosclerosis and Buerger’s disease (the latter, an inflammatory blood vessel condition brought about by prolonged tobacco use and previously curable only by amputating the affected limb or limbs). HGF targets hepatocytes and other epithelial cells inducing them to regenerate and grow blood vessels within affected tissue. The drug is administered by injection and is in global late-stage clinical trials where it is demonstrating its efficacy.
Bluebird Bio describes itself as a clinical-stage biotechnology company with its headquarters in Cambridge, Massachusetts. It is using gene insertion technology to target beta-thalassemia, adrenoleukodystrophy, retinal disease, chronic lymphocytic leukemia, Hemophilia B and Parkinson’s Disease. It has developed three products. One is Lenti-D which is currently in Phase 2/3 clinical trial for treatment of childhood cerebral adrenoleukodystrophy (CCALD). The second, LentiGlobin is in Phase 1/2 trial for treatment of beta-thalassemia (sickle cell disease). And its third, Chimeric Antigen Receptor or CAR is in preclinical development for the treatment of leukemia and cancerous tumors. All treatments involve modifying the patient’s own T Cells to address the genetic reason for the disease.
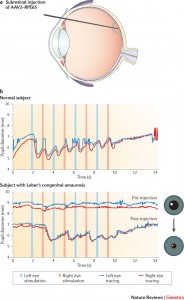
Children’s Hospital of Philadelphia (CHOP) has developed a treatment for inherited retinal degeneration, Leber congenital amaurosis (LCA), a very rare form of early onset blindness. The Center for Cellular and Molecular Therapeutics at CHOP uses a genetically engineered adenovirus to carry a normal verison of the gene, RPE65 to repair mutated versions in patients suffering from early onset blindness. They have designated the product AAV2-hRPE65v2. The first clinical trials as reported in 2009 involved 12 patients, ages 8 to 44. Although full eyesight was not restored, 6 patients vision improved enough to no longer classify them as legally blind. Functional improvement was obtained within two weeks after a single injection of genes to produce the proteins needed to make retinal light receptors switch on. Since these early trials CHOP has entered Phase 1/2 clinical studies with a plan for a Phase 3 study prior to getting the product approved for the European Union and the United States. This is expected in 2015.
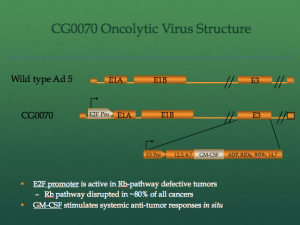
Cold Genesys is an Irvine, California biotechnology company developing innovative cancer immunotherapy for the treatment of Non-Muscle Invasive Bladder Cancer (NMIBC). Their product, CG0070, is delivered using an adenovirus containing a distinct retinoblastoma gene (RB) disruptive pathway. The adenovirus multiplies within the cancerous cells and delivers its load causing the cells to die. The adenovirus also sets of the immune system using a powerful stimulus called GM-CSF, a cellular signaling molecule. The molecule attracts cells from the immune system to attack the cancer site. Currently the technology is undergoing Phase 2/3 trials.
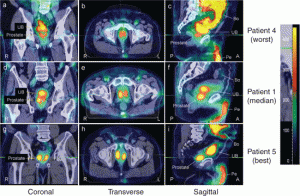
Henry Ford Hospital, in Detroit, Michigan is in the process of testing its 2nd generation gene therapy products in clinical trials to treat recurrent and newly-diagnosed cases of prostate cancer. In Trial 4 a new and improved adenovirus to deliver the hNIS gene to patients with cancer. The adenovirus is designed not only to attack cancerous cells but also can be watched (see image below) while it works inside the patient using routine medical imaging. The patient receives a radioactive substance commonly used in thyroid scans. Oncologists can study optimal placement of the virus within the prostate gland and can watch how long the virus remains active and how well it spreads. Results have been very encouraging.