October 8, 2015 – In the past five years 33% of all new medicines approved by the American Food and Drug Administration have focused on rare disorders often called “orphan diseases.” A category that numbers approximately 7,000, “orphan diseases” impact 350 million people around the planet. The best known is cystic fibrosis. For a comprehensive list, however, you can visit the Global Genes website where they appear alphabetically.
Orphan diseases follow the 80/20 rule. That is 80% are genetic in origin. And the 80/20 rule works as well in identifying the most common of them with 350 of them classified within the 80%.
Orphan diseases impact the young. Approximately 50% of them happen to children with 30% of those afflicted never seeing their 5th birthday. Orphan diseases cause 35% of all deaths in the first year of life.
Until 2008 very few new drugs had been developed to combat these diseases since bio-pharmaceutical companies focused largely on common diseases. But the past seven years have seen changes. The industry has discovered this market and is pouring research and development money into what was of marginal interest in the past. Why? Because of the decoding of human DNA and because of advances in computing and artificial intelligence.
One orphan disease project is compiling a database of DNA patients with rare diseases to aid in diagnosis and research. Located in the United Kingdom it is called the 100,000 Genomes Project. The plan involves creating a genomic medicine service for the general public covering from diagnosis to treatment. Initial enrollment – 75,000 people in total with 60% suffering from an orphan disease. The response by patients and affected families has been extraordinary with thousands offering to have their DNA tested.
In the United States the Food and Drug Administration is speeding up the process of approval for drugs aimed at orphan diseases. Why? Because the industry likes the payback with treatment running into hundreds of thousands of Euros and U.S. dollars annually.
Just two examples. A Dutch biotech firm, UniQure, has launched a new drug to combat lipoprotein lipase deficiency at a cost of 53,000 Euros per vial equating to a one year treatment equaling $1.1 million Euros or $1.4 million U.S. for 42 injections. An American company, Alexion has developed a medicine to treat atypical haemolytic-uremic syndrome, a kidney disease, at a cost of treatment of $400,000 per year.
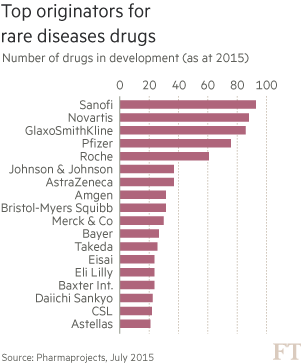
For a list of bio-pharmaceutical companies jumping on the orphan disease bandwagon, check out the graphic below.
As genomics advances it is expected that personal disease treatment will become the norm with designer drugs developed for individuals. This should provide an impetus for governments and insurers to drive down the price for treatment because every disease will be considered personal and therefore rare.