April 22, 2016 – One of the biggest challenges for battery designers is choosing materials that can withstand the repeated discharge and recharge cycles. All of us who have used laptops and cell phones for years no exactly what happens to lithium-ion batteries at a certain point. They simply become less reliable after hundreds of discharge-recharge cycles.
This is one of the many challenges faced by electrical vehicle manufacturers like Tesla, Faraday and General Motors. Lithium, the anode material of choice has demonstrated discharge-recharge cycle limitations in the tens of thousands. To meet the same longevity found in vehicles powered by internal combustion engines, cycles in the hundreds of thousands would be highly desirable. This would give EVs and all the other battery-powered devices we use today a power source that would never need replacement.
So researchers have been playing around with lots of different kinds of materials to create batteries that don’t degrade or lose their ability to accept an electrical charge. At University of California Irvine, one team thinks they may have found the answer. It is a battery made of nanowires, thousands of times thinner than a human hair. A bunch of them put together creates a very large surface area that can be stored in a small space allowing for a significant amount of onboard electron storage.
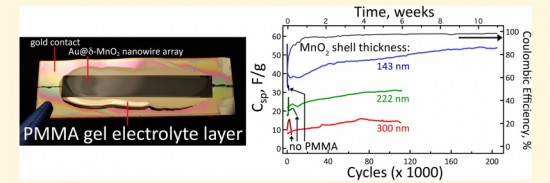
Nanowire filaments when exposed to discharge and recharge cycles typically will degrade and crack. This is the overriding material challenge. The researchers chose gold nanowire as the anode material and combined it with a coating of manganese dioxide and a methyl methacrylate electrolyte (PMMA) plexiglas-like gel. The nanowire battery is seen below in the image on the left. Test results done over three months involving 200,000 discharge and recharge cycles appear on the right. What they show is a battery that does not lose capacity nor structural integrity over an enormous amount of reuse. In addition the battery is flexible which makes it ideal for multiple form factors.
A paper appearing in the journal, American Chemical Society’s Energy Letters, describes how the University team achieved a nanowire-based battery and capacitor capable of providing “extremely long cycle lifetimes.”
Just how different is this new battery?
Most current storage technologies “typically die in dramatic fashion after 5,000 or 6,000 or 7,000 cycles at most,” states Reginald Penner, Chair of the Chemistry Department at University of California Irvine. But in coating the nanowires with the PMMA thin gel layer the cycle capacity can now be extended to “hundreds of thousands of times without losing capacity.” It appears that the gel is the key because it prevents the cracking of the nanowires and gives the structure great flexibility.
The next step is to continue to test this breakthrough storage technology and then determine how to move it from the lab to commercial production.