The FDA in the United States has approved the Argus II Retinal Prosthesis System, the first bionic eye, an artificial retina for sufferers of Retinitis Pigmentosa (RP), a disease that affects 1 in 4,000 in North America. It works by stimulating retinal cells with electrical signals to create visual patterns which the device wearer can then interpret as sight.
RP is a degenerative eye disease affecting the retina. The damage slowly diminishes a person’s ability to see eventually leading to complete blindness. It can be genetic and run in families. Early symptoms may appear in childhood usually with night and low light vision loss. As the sufferer ages they experiences blurring of vision, an inability to see colour, tunnel vision or loss of central vision, eventually an ability to see only bright flashes of light, and finally total blindness.
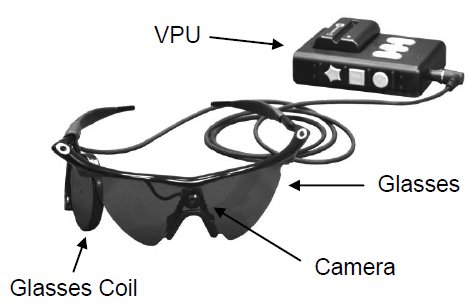
The development of the Argus II, by Second Sight, a company located in California, provides RP sufferers with bionic vision using images captured on a miniature video camera mounted in a pair of special glasses that include a wireless transmitter. The wearer straps on a video processing unit (VPU) at the waist.
The VPU processes and transforms images it receives from the camera through a thin-wire cable that runs from the waist pack to the wireless transmitter which then communicates with a microelectrode array called an epiretinal prosthesis, a surgically implanted device, seen below, which is placed on top of the wearer’s retina. The electrical pulses received by the prosthesis bypass the damaged photoreceptors stimulating the remaining undamaged retinal cells which transmit information to the brain through the optic nerve. The current Argus II wearer doesn’t see with the same level of acuity as a person with normal vision. But they can see pixels of light with detail enough to recognize a face or navigate through busy city streets. 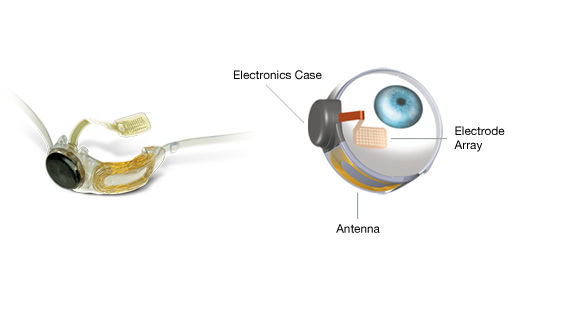 The Argus II is a major breakthrough for RP blindness. But Second Sight has future plans to develop electrodes that can be implanted in the brain to create enhanced bionic vision capability. Although the FDA has approved the Argus II for RP sufferers in the United States,the device is already being used to treat other causes of blindness in Europe.
The Argus II is a major breakthrough for RP blindness. But Second Sight has future plans to develop electrodes that can be implanted in the brain to create enhanced bionic vision capability. Although the FDA has approved the Argus II for RP sufferers in the United States,the device is already being used to treat other causes of blindness in Europe.










A promising development. I’m wondering about the cited 1/4000 frequency? I have a couple of elderly friends that have it severe enough that they can’t drive. At age 74 I’m in the early stages, but given enough light I can still read and drive well enough. I have difficulty driving at night. One of my friends, a professional philosopher and writer, is 76. His condition started when he was 65. He now has great difficulty reading ordinary trade books, and doesn’t drive. My other friend is 87; he can still read pretty well, but he shouldn’t drive. My mother is 97; she has it so severely that she can’t read my letters in 16-point type. I’m not arguing my personal experience is statistically significant, but I’m guessing at least 10% of the general population over the age of 70 has it to some degree. Maybe the electronic eyeballs will improve at a rate that will keep up with the deterioration in my eyes. Borg eyes would beat the hell out of blind eyes.