April 27, 2017 – We haven’t known about stem cells, which some refer to as the god cell, for very long. A paper in Nature, published in February of 1963, described a type of cell that we would call stem cells today based on observations made by the researchers who described the following: when these isolated cells were transplanted into a heavily irradiated mouse, ostensibly to kill its own cell population, the introduced cells proliferated and differentiated to form colonies of various tissue types.
But 1963 may not be the earliest date in which a cell exhibiting stem cell characteristics is mentioned. The term is mentioned in a 1932 medical journal, but the reference to what we know as stem cells today seems inappropriate. And then there are older references to stem cells back to 1905.
One thing is clear, however, we had little understanding of what these cells were and their potential.
Today we know about stem cells. The Mayo Clinic’s website calls them the “body’s raw materials,” cells from which all others can be generated. But what makes them so different from all those other cells in our bodies? Our bodies contain 200 known varieties of cells. Each, with the exception of blood cells, has a nucleus, mitochondria plus other organelles, a common chemical soup, and consistent structural features.
Yet there is no such thing as a typical cell. All cells pretty much do the same thing. They divide until their ability to do it stops. At that point, they die. We lose 96 million cells per minute but we also gain that same number in the same amount of time. The behaviour of each cell is determined by instructions given by genes located on chromosomes within the cell nucleus.
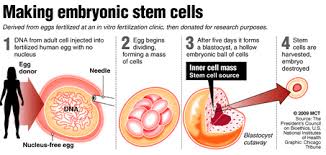
When we humans start out, our first cell is a fertilized egg combining DNA from both of our parents. The egg divides to eventually form a ball of cells we call an embryo. And the modern story of stem cells begins with what scientists first discovered about embryos. They contained cells that were non-specific but capable of being instructed to differentiate to turn into us, producing our bone, muscle, organs, brain, nerves skin, and nails. With all that differentiation leading to the 200 known varieties of cells we find in our bodies today, scientists overlooked one. Stem cells didn’t cease to exist when the embryo began to differentiate. They very much exist within all those differentiated organs and tissues that form us. You can find them in muscle, bone, organs, and fat. In fact, body fat may prove to be the best source of harvestable stem cells that we can use to cure so many of the chronic conditions and diseases we humans encounter.
Most of us when we think of stem cells, we think embryonic. That’s because that’s where the science of stem cells first started. To make embryonic stem cells researchers use a technique called somatic cell nuclear transfer. It involves manipulating donated eggs by removing the original DNA contained in the nucleus and replacing it with DNA from a donor adult cell. Cell division then follows until an embryo forms. The embryonic cells, about 100 of them on maturity, are then harvested and the embryo destroyed.
It is this use of human embryos that has created the controversy associated with stem cell research. For those who aspire to the belief that life begins at conception, harvesting and destroying an embryo is seen as murder. Lobbying by religious groups soon led to restrictions in the science in the United States with only a limited number of embryonic stem cell lines allowed.
But the promise of these cells and their ability to differentiate into any type of tissue has kept research laboratories busy in places other than the United States where more than 300 existing lines of human embryonic stem cells being cultivated in Canada, Sweden, France, Spain, and the United Kingdom. These lab-grown embryonic stem cells represent one branch of stem cell research. And because the cell populations are artificially cultured in laboratories there are some significant problems that can happen.
Consider evolution, the engine that drives speciation. From reptiles came dinosaurs, mammals, and birds. How did that happen? Mutation. And mutation worked best when there were large populations of individual species who through breeding, living and dying further differentiated and survived.
Now apply that same observed principle to laboratory populations of continuously dividing limited lines of stem cells. Just like in an isolated population of animals with a limited amount of DNA to share, mutations can produce undesirable results. In the case of laboratory-grown stem cells, a cell meant to cure could because of undesirable changes kill.
In addition, growing a large enough population of embryonic stem cells to cure people is an expensive proposition. The science, therefore, needs to find a better way to use the potential regenerative capacity of these cells.
Finding a new source of stem cells is critical. And lo and behold they are there, in our bodies, lurking among all those differentiated cells which came after conception.
In Part 2 we’ll continue the story, describing how readily available stem cells existing within us represent a medical revolution in tackling a wide variety of diseasee challenges from autoimmune to cardiac, hepatic, pancreatic, gastrointestinal, respiratory, neurodegenerative, ophthalmologic, spinal cord, skin, osteo, and more.










[…] https://www.21stcentech.com/god-cell-story-stem-cells-regenerative-medicine-part-1/ […]