October 23, 2013 – A French company, CARMAT, has recently received approval to begin transplanting its new bioprosthetic artificial heart into patients suffering from terminal heart failure. The initial trial involves four recipients. Three hospitals are involved, the Georges Pompidou European in Paris, the Marie Lannelongue Surgical Center in Plessis-Robinson, and the Laennec-Nord University Hospital in Nantes.
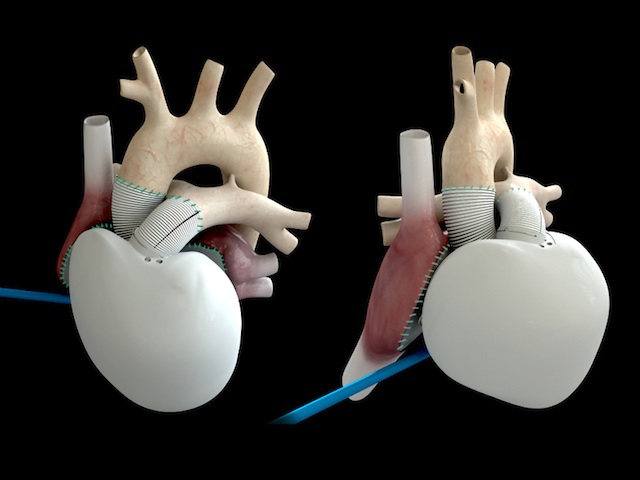
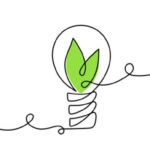
The CARMAT weighs 900 grams (1.98 pounds). It is made from synthetic and biological materials and contains two ventricles (the equivalent to the two lower pumping chambers of the human heart) separated by a sterile, chemically treated calf membrane. Two motorized pumps push blood from the ventricles to the aorta and pulmonary artery. The valves that control the flow are also made from biological materials. Embedded sensors regulate the heart’s function adjusting the heart rate based on the levels of exertion performed by the recipient. A wearable external lithium-ion battery pack provides the power. In addition the transplant recipient wears a belt that sends data to the hospital team to monitor vital signs and the operation of the device. The CARMAT has a 230 million beat life equivalent to about 5 years of operation. The heart prior to human clinical trials has been tested on calf hosts. Cost for each CARMAT including the operation is estimated at $218,000 U.S.
In past postings I have written about CARMAT and other artificial hearts such as the SynCardia, HeartMate II and BiVACOR. Of all of the current artificial heart products it is the only one that uses the combination of synthetic and biological materials making it less likely to cause blood clots to form. That’s because the CARMAT uses hydrophobic materials which are smooth. This discourages adhesion of blood cells which can lead to clot formation. Clots can cause strokes and blockages of blood vessels.