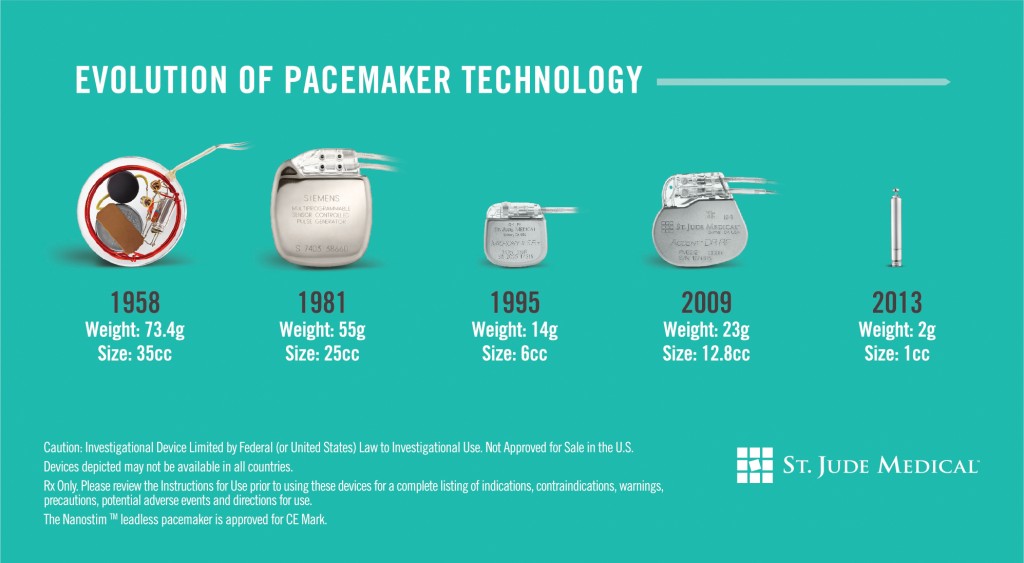
October 24, 2013 – St. Jude Medical, located in St. Paul, Minnesota, has acquired Nanostim Inc., a California developer of a tiny wireless pacemaker that is different from any other pacemaker product on the market. The Nanostim (TM) is 10% the size of current pacemaker technology, weighs 2 grams and is 1 cubic centimeter in size. Compare it on the far right in the illustration below to the pacemakers that preceded it.
Traditional pacemaking technology requires a surgical procedure in which pacemaker leads are threaded through veins to attach to the heart muscle while the pacing device with battery pack gets inserted under the skin into the chest of adults, and the abdomen of children.
But that is not how you implant the Nanostim. No surgery is required. Instead a minimally invasive procedure is employed called a catheterization. Catheterizations today are commonly performed in place of many surgical procedures. For example catheters deliver stents to open blocked coronary arteries, implant heart valves (my daughter’s pulmonary valve was inserted this way), and close holes in the heart by inserting devices that resemble butterflies. And now with Nanostim they are delivering pacemakers.
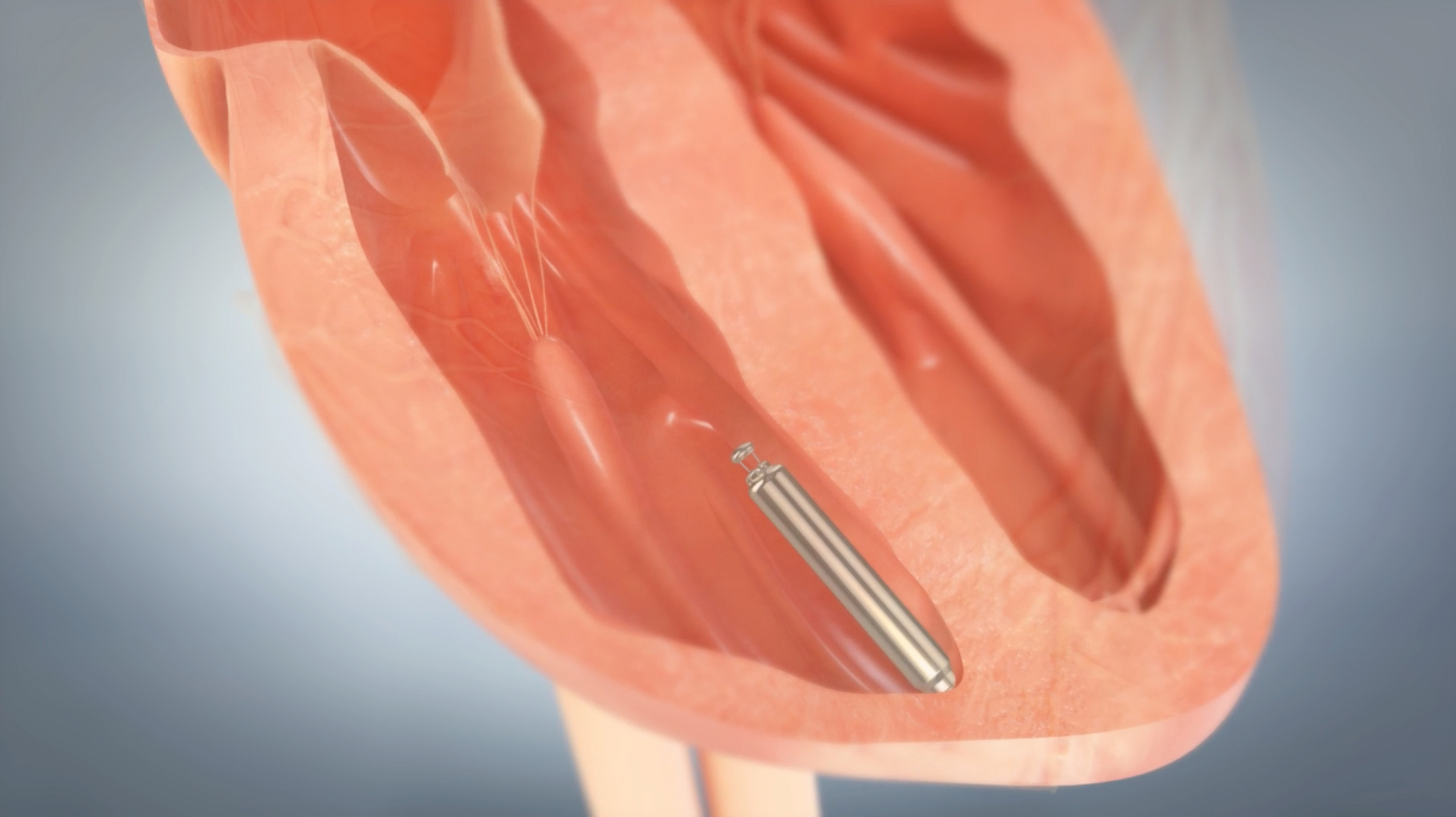
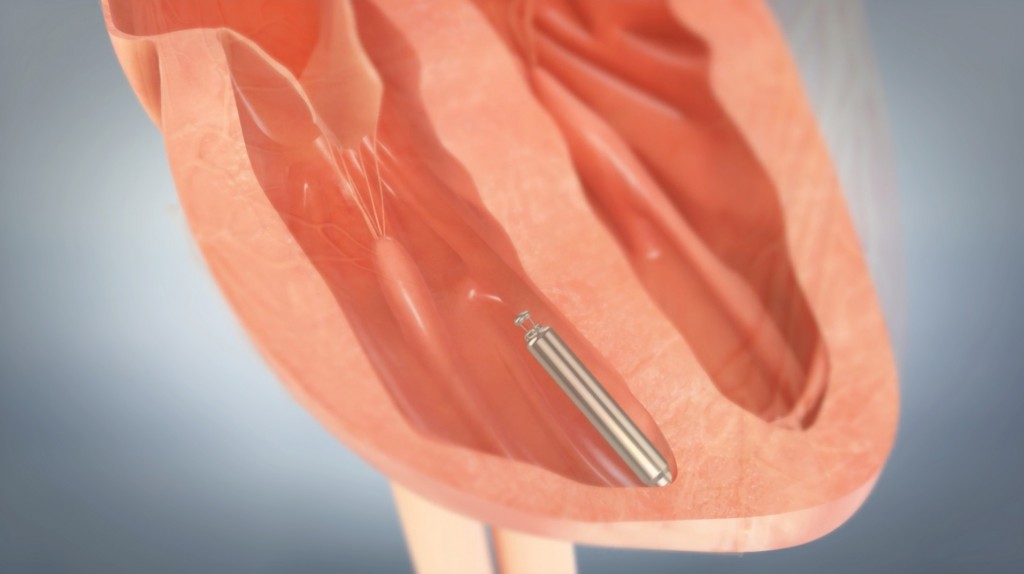
Nanostim is inserted and placed in a ventricle (a lower pumping chamber of the heart as seen in the illustration below.) Once implanted its battery is rated to last between 9 and 13 years. It requires no surgery either for implanting or extraction and replacement when needed. European clinical trials have been very successful and as a result European Union approval has been given to roll the device out for general population use. We should see Nanostim in North America in a couple of years.
Today on average 700,000 heart pacemakers are implanted globally each year. The frequency of “complications” for the procedure and post-procedure is estimated to be between 10 and 59% depending on the definition of “complication.” This can include pacemaker malfunctions leading to symptoms such as light headedness, dizziness, fatigue, slow heart beat and more. Other complications include post-operative bleeding and infections both in the device as well as the leads. Also because leads are threaded through veins that get close to the lungs there have been incidents where leads have perforated a lung leading to collapses either during the procedure or after because the leads are not properly anchored to the heart.
So Nanostim can be described as one of those technologies that is truly a positive disruptor for the field of cardiology. This is a welcome technological advancement.






According to Medtronic, Inc., they have joined Nanostim with their own implantable pacemaker: the Micra Transcatheter Pacing System (TPS). In a December 9, 2013 press release the company announced a successful Micra TPS implant in a patient living in Linz, Austria as part of a clinical trial. Introduced using a catheterization procedure, the device has all the benefits of the Nanostim. The clinical trial will enroll up to 780 patients in 50 centers. The company intends to post the first 60 results with 3 month follow up after mid-2014.