June 8, 2019 – Dr. Deepa Ghosh and a team of researchers from the Institute of Nano Science and Technology in Mohali, Punjab, published a paper in April of this year in the journal ACS Applied BioMaterials, where they described the invention of a hydrogel designed to provide long-term survival of mesenchymal stem cells (MSC), adult stem cells that can be harvested from a patient, and used in a growing number of medical treatments.
I have a personal interest in MSC for use in treating osteoarthritis, something I have suffered from for more than 45 years. I have always been concerned about the viability of stem cell injections particularly in terms of rates of survival once transplanted into an injury site. One reason for concern is how the cells will defend themselves from the body’s natural immune system. MSC implants could be subject to attack from T-cells and other white cells that would treat their arrival as a foreign substance.
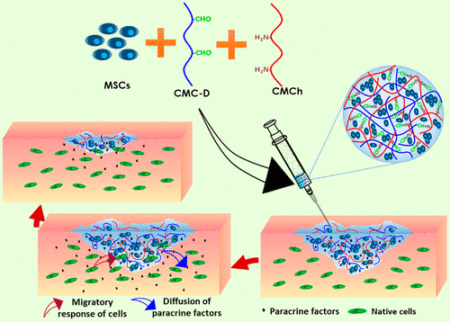
But what Ghosh and her colleagues have created is a protective transporting hydrogel shell that contains the MSC supporting the viability of the cells for as much as a month after injection. The hydrogel is a concoction of cellulose-like material and chitosan, a derivative of chitin which forms the exoskeleton of crustaceans like shrimp.
In an article appearing in yesterday’s The Hindu Business Line, Ghosh is quoted stating, “the hydrogel addresses the issue of the long- term survival of adult stem cells in simulated cultures that mimic the actual body tissue. We observed that cells survive and multiply for a period of one month, which is sufficient time for tissue regrowth.” In other words, the hydrogel doesn’t interfere with the capability of the MSC to interact with neighbouring cells and stimulate division.
Ghosh’s hydrogel, following implantation, supports normal functioning of the MSC contained within it. Subsequently the MSC secrete a growth factor called paracrine which signals nearby non-stem cells to alter behaviours. MSC injected into a knee joint protected by the Ghosh hydrogel would, as a result, in no way inhibit signaling to chondrocytes, cartilage cells, or osteocytes, bone cells, to begin to multiply. It is interesting that the two cells tested by Ghosh and her team were chondrocytes, and fibroblasts, the latter skin cells, and showed no inhibiting factors. The next step will be to apply what they have learned in the laboratory to animal studies. And if those are successful we should see human clinical trials in the near future.
The potential of the hydrogel-MSC composite injection serving as scaffolding for reconstructing knee cartilage or repairing wounds and damaged organs cannot be underestimated. The injectable’s ability to take the required shape within an injection site and provide adhesion to the surrounding tissue is a significant breakthrough that if replicable in animal and subsequent human patients, could change the way osteoarthritic joints and other injuries get treated in the near future.