July 31, 2016 – Today lithium-ion batteries are the mainstay of power storage for smartphones, computers and electric vehicles (EVs). The pros for lithium batteries remain the same. They deliver lots of juice in proportion to their weight. The cons though are greater. Lithium-based batteries run hot (even catch on fire). They degrade in terms of storage capacity over a relatively short lifetime. Don’t believe me? Just consider your smartphone battery and tell me after a year if it still holds its charge to the same extent as when you first got it. And finally lithium isn’t exactly the most abundant substance on the planet so relying on it for the long term as we transition from a carbon-based economy to a zero emission future is somewhat problematic.
But as long as we continue to rely on lithium’s energy pop we can make improvements to the technology. That’s what a group of MIT engineers have proposed in a new lithium-based invention which they call the nanolithia cathode battery. In a paper entitled, Anion-redox nanolithia cathodes for Li-ion batteries, appearing in the journal Nature Energy, authors Zhi Zhu, Akihiro Kushima, Zongyou Yin, Lu Q, Khalil Amine, Jun Lu and Ju Li describe the severe challenges that conventional lithium-ion batteries display. They explain the voltage gap between charge and discharge in the current technology. And they describe the instability of the electrolytes and expensive membranes required to make these batteries work.
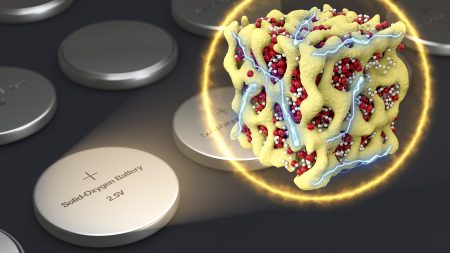
That’s why they have developed what they call an “oxygen anion-redox cathode” that is stable and can be incorporated into a fully-sealed battery with little in the way of energy loss over its lifetime.
How do conventional lithium-ion batteries work?
They take oxygen from ambient outside air to create a chemical reaction with the lithium to produce electricity. And when charging they release the oxygen back into the air.
How does the nanolithia battery differ?
It doesn’t draw outside air which means it doesn’t have to deal with removing carbon dioxide and water vapour, both killers to conventional lithium-ion technology. Instead the nanolithia battery draws on oxygen stored within it. The oxygen combines with lithium transforming into three different solid chemicals that form a glassy compound. With no material phase changes from solid to gas and back again, this battery technology doesn’t deteriorate or lose storage capacity. It doesn’t need the extra hardware to scrub the carbon dioxide and remove water vapour. Its heat dissipation is considerably reduced, just 8% of that found in current lithium-ion technology. You can’t overcharge it which can cause a lithium-ion battery to explode. And it retains and delivers energy better and recharges faster. The secret is in the “nano” matrix of tiny particles embedded with cobalt oxide. The result, a battery by weight which can store twice the energy per kilogram of the current technology.
Is the battery scalable? Is the battery more expensive than existing lithium-ion technology?
The challenge for anything invented in a laboratory setting is can it be commercialized and from a cost perspective improve upon the current industry standard. The inventors say yes to both points. States Xiulei Ji, in the Chemistry Department at Oregon State University, and not one of the authors of the above referenced paper, “this is a foundational breakthrough, which may shift the paradigm of oxygen-based batteries.” The inventors are more modest in representing their invention. They state in their conclusion that “our nanocomposite still has much room for improvement.” They believe they can increase the energy capacity to weight and want to experiment with other materials to make the battery even lighter while retaining the same sealed characteristics that make it more suitable as a solution for transitioning from carbon-based energy to zero emission technology using electricity generated by advanced storage batteries.