April 24, 2017 – The news that a malaria vaccine is ready to be administered to the public in areas prone to the disease is exciting but there are some limitations in its present form. The vaccine, known as RTS,S/AS01, or Mosquirix (TM), is not a single dose treatment. It must be administered four times, three monthly doses to begin, followed by a fourth given 18 months later. In clinical trials, this regimen has been controllable. The question is can it equally be effective in the real world.
According to the World Health Organization’s Regional Office for Africa (WHO/AFRO), Mosquirix is the furthest advanced malaria vaccine at present. There are others under development as well but none ready for widespread public use. The vaccine will provide partial protection against malaria in young children helping them to build immunity against five species of Plasmodium, the protozoan parasite responsible for the disease.
In Africa, Plasmodium falciparum, Plasmodium malariae, Plasmodium vivax and Plasmodium ovale, are the known agents of infection. Other human malaria protozoans include Plasmodium knowlesi which is found throughout Southeast Asia. Some of the Plasmodium parasites have an ability to hide in the body after an initial infectious stage, causing relapses months and even years later. The administration of the vaccine over a period of 21 months may be one way to ensure permanent immunity protection from the disease. And researchers are looking at repeating the dose beyond the 21-month protocol if needed.
Administration of Mosquirix will start with pilot programs in Ghana, Kenya, and Malawi. It is hoped in the period from 2020 to 2022 that the results of the pilots will make it feasible for wider usage in Africa and other areas where malaria remains an ongoing threat.
The vaccine’s development owes a debt to the Bill & Melinda Gates Foundation, the PATH’s Malaria Vaccine Initiative, headquartered in Seattle, the Walter Reed Army Institute of Research, headquartered in Bethesda, Maryland, and the biopharmaceutical giant, GSK. Clinical trials have been conducted in seven countries in Africa with the vaccine given to almost 15,500 children. In these clinical trials immunized children experienced 46% fewer cases of malaria when compared to other trial vaccine products. Noted in the results has been a decline in efficacy over time which has led to suggestions of repeating the administration of Mosquirix periodically. Also noted, treatment has included other forms of malaria protection such as insecticide-treated bednets which were used by nearly 80% of trial participants.
The Lancet published final study results in a paper released in April 2015 that demonstrated effective immunization against malaria in 39% of those treated. The waning of the vaccine’s effectiveness within the current four dose protocol has researchers looking at repeated periodic administration as a potential method of keeping the disease at bay.
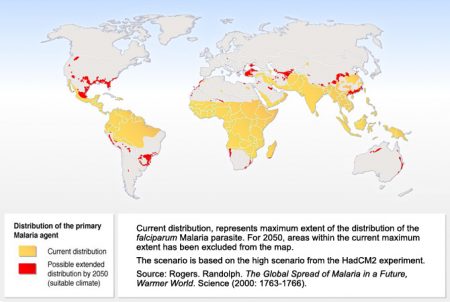
States Dr. Seth Berkley, Chief Executive of Gavi, an organization helping to fund the Mosquirix pilot program, “The world’s first malaria vaccine is a real achievement that has been 30 years in the making.” He notes that the importance of its success will lead to a global distribution of Mosquirix helping to defeat the scourge of malaria which inflicts “a terrible burden on many of the world’s poorest countries, claiming thousand of lives and holding back economies.” Today the number of annual deaths from the disease total 438,000 with hundreds of millions more infected. And when you consider how climate change is widening the area where malaria-bearing mosquitoes can thrive, including migration into more temperate latitudes, it will become a potential threat to populations in the Developed World as well.