Semaglutides have become all the rage for people trying to lose weight. It is prescribed under the brand names Ozempic, Wegovy and Rybelsius. Novo Nordisk is the manufacturer. Developed to address the management of Type 2 Diabetes, a welcomed side effect has been weight loss.
Ozempic and Wegovy are self-administered injectables. Rybelsius is a pill. The drug classification is called a Glucagon-Like Peptide-1 Agonist. These are administered to patients along with advice to use with dieting and more exercise. These drugs control blood sugar levels making them effective for the treatment of diabetes. They can also be used to prevent major cardiovascular events such as heart attacks and strokes.
Earlier this year I was diagnosed as pre-diabetic. My blood sugar levels were above the normal range. In the three-plus years of COVID-19, I had gained over 15 kilograms (33 pounds). That’s when my new and very young family doctor offered to put me on Ozempic telling me she was seeing great results in terms of weight loss and reversing blood sugar levels. I knew people who had been put on Ozempic after being diagnosed with Type 2 Diabetes. They described the side effects which included significant weight loss along with some very negative effects. They suffered from nausea, extreme bowel discomfort, vomiting, diarrhea or constipation. I politely told my doctor, no thanks. Instead, I went on a diet and exercise program and have shed all the weight in the last six months. I am no longer pre-diabetic.
Why Has Semaglutide Taken the World by Storm?
The World Health Organization (WHO) describes obesity as one of the most neglected public health problems. Being overweight is malnutrition’s opposite of which the latter is related to food insecurity. But obesity which the WHO calls globesity is a growing threat not just to Global North countries but also to those in the Global South.
Close to 2 billion out of nearly 8 billion today on this planet are considered overweight with 13% of adults classified as obese. In the United States today, 34% of adults and 15 to 20% of children and adolescents are obese.
Diet and exercise, plus a plethora of pills have been the traditional ways humans have approached weight loss. This has spawned a global weight-loss industry which in 2021 was valued at US $224 billion annually with projected growth to $405 billion by 2030.
Semaglutides have made Novo Nordisk a very rich company and possibly the biggest disrupter to the weight-loss industry. The side effects, however, remain the biggest inhibitor to growth in use. Based on current sales, however, it appears doctors and patients have fully bought into using semaglutides to drop significant body mass.
A Therapeutic Weight Loss Nanogel Alternative
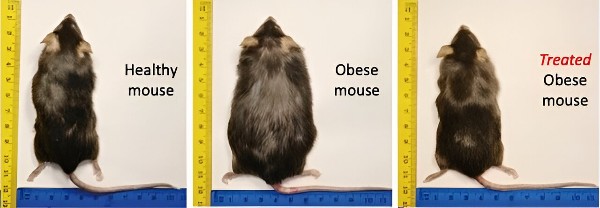
At the University of Massachusetts Amherst, S. Thai Thayumananavan, Professor of Chemistry and Biomedical Engineering, has invented a treatment for obesity and Type-2 Diabetes using a nanogel infused with a synthetic thyroid hormone or thyromimetic. Thyromimetics are drugs first developed to treat liver disease. But in this case, they are being used to treat obesity, high cholesterol and metabolic disease.
The test subjects for the study were mice fed a high-fat diet for weeks. They soon became obese. When given the thyromimetic packaged inside a nanogel and delivered daily by intraperitoneal injection (the needle goes directly into the liver, a much deeper injection than a typical vaccine) the mice quickly lost weight even though they were still being fed a high-fat diet.
Delivered this way, the thyromimetic activates the beta receptor hormone which causes fat oxidation, reverses cholesterol levels and increases metabolic rate. Originally intended for liver disease treatment, the results point to a “nanopartical-mediated pharmaceutical” alternative for treating weight loss.
For this to become a weight loss treatment, the delivery mechanism will have to be less invasive than intraperitoneal injection. If this issue can be solved, then move over semaglutide, you have a strong competitor in the wings.
The study results were published in August this year in an issue of PNAS Nexus, a journal of the National Academy of Sciences of the United States.