January 23, 2015 – Carbon capture and sequestration, or CCS, is the engineering answer to “having our cake and eating it too!” Those who believe we can reduce CO2, by capturing it as it is produced, and then piping it deep into the Earth’s crust where it bonds with rock permanently, will not be happy with the latest research from Massachusetts Institute of Technology.
An MIT team from the Department of Earth, Atmospheric and Planetary Sciences, studied how CO2 reacts with native rock when injected into the ground. What they found was that only a small percentage of the gas bonds permanently while the rest remains gaseous or liquid. In the case of the latter two, the CO2 can find its way back through cracks to the surface and out gas back into the atmosphere. Their research results appear in the latest edition of the journal, Proceedings of the Royal Society A.
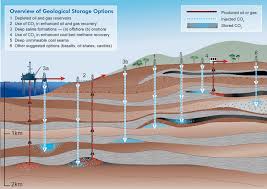
Typically carbon sequestration uses deep saline aquifers and gas fields like those depicted in the above image. The CO2 is concentrated and liquified before injection. Once it is trapped the injected fluid percolates into the rock, typically sandstone, where it can undergo a chemical alteration in association with the rock’s chemistry. But no rock formation is uniform. There are cracks and small pockets where the CO2 can remain as a gas with no bonding or chemical alteration taking place. This means within the underground sequestration field solidification rates are likely much lower than theorized by those who practice CCS.
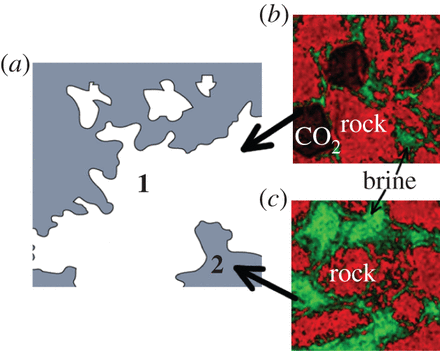
The illustration above appears in the study and shows how CO2 when sequestered can move from a (a) briny solution in the rock to two end results. The (b) result in the upper picture on the right shows a combination of gaseous CO2 and brine-laced CO2 , while the (c) result on the lower right shows the CO2 remaining in the brine but separated from the rock in which it is sequestered.
In their conclusions, the researchers, Yossi Cohen and Daniel Rothman, geophysicists, state:
“Our results suggest that only a small fraction of the injected CO2 is converted to a solid mineral. The remainder stays in its dissolved ionic form or is trapped in its original form. Whether a domain will go into dissolution trapping or mechanical separation depends on the concentration gradients, the properties of the rock and the porosity available for transport.”
Prior to this research the expectation among CCS engineers was that most of the CO2 when interacting with deep rock formations solidifies. Cohen and Rothman state, “Our work suggests that significantly less will precipitate.”
So if CCS isn’t the engineering panacea to reduce CO2 from human-generated sources it would seem that reducing energy from emission-generating fossil fuels represents the best way for us to decrease the global warming impact. To meet growing energy needs our focus, therefore, must be on rapid expansion of CO2 neutral renewable energy sources. We can’t continue to “eat the CO2 cake” without dire consequences.