An antibody infusion therapy that can treat multiple variants of COVID-19 is likely in the near future to move from emergency authorization use in the U.S. to being given the green light for widespread intravenous administration in fighting the disease.
Antibody research going back to 2003 during the first SARS COVID pandemic has helped lead to the discovery of a number of candidates capable of being turned into therapeutic treatments. But only now has one specific one emerged as a monoclonal therapy to treat patients after getting COVID-19 and even potentially as a preventive.
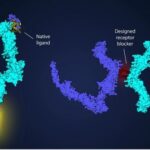
A Lawrence Berkeley National Laboratory structural biologist, Jay Nix, in performing X-ray crystallography on tissue samples from COVID-19 survivors is getting the credit for creating structural maps of the virus along with the identification of the natural antibodies able to bind to it.
Singling out the most promising antibodies of which there are a number has led to the emergence of S2H97 which has been given the drug name Sotrovimab. In clinical trials both in the United States and Europe, intravenous administration of 500 milligrams of S2H97 has demonstrated effectiveness in treating people with mild to moderate COVID-19 infections, producing 85% hospitalization rate reductions over patients receiving a placebo.
In a subsequent animal study given prophylactically to hamsters, S2H97 has demonstrated its ability to be a COVID-19 preventive. Obviously, human follow-up studies are needed before we can claim it to be a COVID-19 stopper.
The question of multi-variant effectiveness has been part of follow-up studies. These have further confirmed the efficacy of this antibody because of the way it attaches to COVID-19 viruses. It appears to target a surface area that remains mutation resistant.
The journal Nature published an article on July 14, 2021, authored by Tyler Starr Nadine Czudnochowski, and Zhuoming Liu from the Fred Hutchinson Cancer Research Center in Seattle, Washington, describing S32H97 and other antibodies and their potency potential to fight COVID-19 in all variants.
Pharmaceutical companies GlaxoSmithKline and Vir Biotechnology have been instrumental in funding the research that has led to Sotrovimab which currently is being administered under emergency use authorization in the U.S. as a single-dose monoclonal antibody for treating mild-to-moderate COVID-19 in both adults and pediatric patients. Sotrovimab was recently authorized for treatment of COVID-19 by Health Canada.