Did life on Earth come from elsewhere? Was our planet seeded by asteroid and comet collisions that produced our early oceans and the chemistry of life within them? The panspermia theory of life’s abundance in the Universe is being challenged by new research on how life’s building blocks came to be on our planet.
A study, originating out of the Center for NanoScience, at Ludwig Maximilians University in Munich, Germany, points to evidence showing gas flows over narrow channels of water could have produced the environment to allow nucleic acids to replicate.
Nucleic acid molecules include deoxyribonucleic (DNA) and ribonucleic acids (RNA). Both store genetic information. Both can replicate this information. Both can mutate and adapt when exposed to external environmental changes.
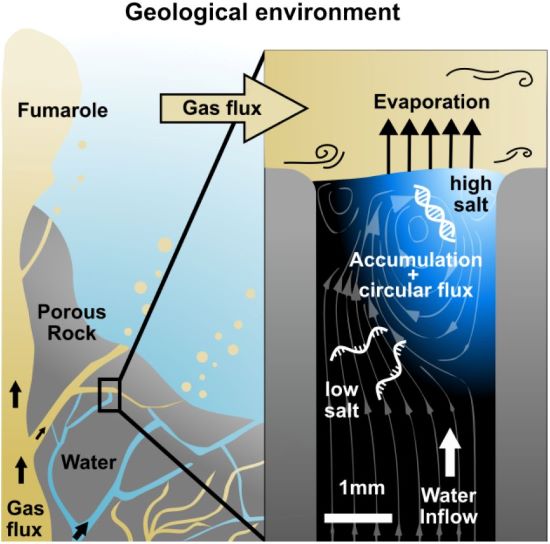
For RNA to replicate into its double-stranded DNA cousin, the molecule needs phase changes within its environment. The environment of early Earth appears to have produced ideal conditions where such phase changes occurred. Volcanism byproducts, gasses, water, and porous cooled lava produced a setting ideal for RNA synthesis.
Philipp Schwintek, lead author in the study, describes how porous rocks and an upward flow of water evaporating while intersecting with gasses being released to the surface produced the necessary setting for RNA synthesis. In a laboratory experiment replicating the early Earth environment, nucleic acid strands begin to rapidly accumulate within five minutes when exposed to water evaporating in the presence of a gas flow. This suggests that a gas/water interface would have been a necessary environmental component for the emergence of life.
Another environmental component for the emergence of life would have been either temperature or chemical changes. In their study, the researchers ruled out temperature changes as a factor. Instead, they looked at changes in environmental chemistry focusing on salt. Salt would readily have concentrated in an environment where gas and water intersected causing evaporation. This would have produced saline brine. Evaporated saline brine is a common source of salt harvested from the ocean today.
With temperature constant in an environment replicating early Earth, water and gas influxes through porous rock produced high concentrations of salt causing nucleic acid molecules to replicate at increasing rates. The molecules observed were multiple strands of DNA. The observations support a hypothesis that life on Earth started here.
Could the same conditions have caused life to emerge in forming planets elsewhere? “Absolutely” state the Munich University researchers. The potential for life to emerge from organic molecules in the presence of volcanism, water and flowing gasses is demonstrable.
Organic molecules don’t necessarily indicate life but their presence provides the necessary building blocks. Are organic molecules rare in the Universe? No, they are quite common.
The Hubble and James Webb telescopes have detected organic molecules throughout the Milky Way and in galaxies far away. Locally, organic molecules and compounds have been found in the gas giant planets of the Solar System and on Mars. The molecules discovered represent the main groups needed for the formation of RNA.
Based on our dating of fossil carbon isotope signatures, life on Earth first appeared 3.7 billion years ago, about 800 million years after the planet formed. We may now have a definitive answer to how it came about.