As the father of a child born with congenital heart disease (CHD), I remember the many medical crises our daughter endured during the first few weeks of her life, let alone the numerous operations and procedures that followed into her 20s. Today, fortunately, she is 40 with a healthy daughter of her own.
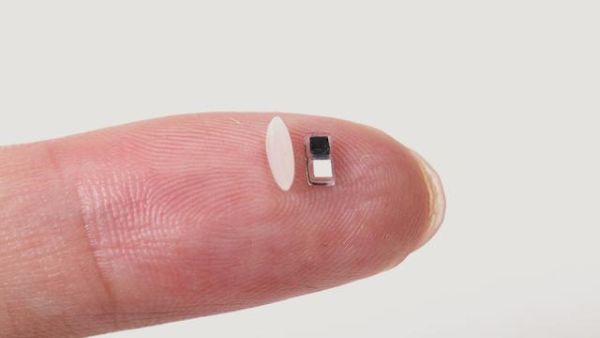
The engineers at Northwestern University have invented a pacemaker smaller than a grain of rice. This device can be injected by syringe into a neonate for temporary pacing when treating several CHDs.
Putting pacemakers in infants is tricky business. One reason is their size. The heart, lungs, and circulatory systems are small, so there is not a lot of room to implant a device. The leads that connect the pacemaker to the heart can often fail.
My daughter’s defect was structural, Tetralogy of Fallot with Absent Pulmonary Valve (TOF/APV) and not electrical. It, therefore, didn’t affect heart rhythm and didn’t require a pacemaker.
What CHDs detected at or before birth, therefore, are best served by a device like this ultra-miniature one?
These are some of the conditions that immediately come to mind:
- If the two lower heart chambers, called the ventricles, produce abnormal heartbeats with prolonged pauses, a pacemaker like this in a neonate would be warranted.
- If operated on shortly after birth, pacing with a device like this could mitigate the risk of the heart stopping and sudden death.
- If the sinus node, located in the left upper chamber, called the atrium, isn’t initiating the heartbeat properly, a pacemaker like this would be warranted.
- If a child has transposition of the great arteries requiring early surgical intervention, post-operative pacing would be temporarily required.
- If a neonate or child before age one experiences borderline heart rates of 50 beats per minute or lower, it could lead to cardiomyopathy and would warrant pacemaking.
The Northwestern device is paired with a wireless wearable that can be applied as an adhesive patch to a child’s chest. The pacemaker is delivered to the surface of the heart by syringe injection. The device is thermostatic and responds to infrared light. When an irregular heartbeat is detected, the wireless wearable on the chest surface emits light pulses that penetrate through skin, muscle and bone to activate pacing.
The pacemaker itself is so tiny that several can be implanted at a time, and using different colours of lights could treat multiple conditions. Designed for temporary use, about a week or so, its biocompatible parts dissolve and are excreted. No surgical extraction is needed.
The journal Nature has published an article on April 2, 2025, describing the technology, and in the abstract it notes that the device “can be readily adapted for a broad range of additional applications in electrotherapy, such as nerve and bone regeneration, wound therapy and pain management.”
John A. Rogers, a bioelectronics engineer at Northwestern, who led the development, told Amanda Morris at Northwestern Now, that the device is designed for pediatric cardiology because it is tiny and minimally invasive for small bodies. He describes how the device works without battery storage, noting, “When the pacemaker is implanted into the body, the surrounding biofluids act as the conducting electrolyte that electrically joins those two metal pads to form the battery. A very tiny light-activated switch on the opposite side from the battery allows us to turn the device from its ‘off’ state to an ‘on’ state upon delivery of light that passes through the patient’s body from the skin-mounted patch.”
His colleague, Igor Efimov, told Amanda Morris at Northwestern Now, “About 1% of children are born with congenital heart defects — regardless of whether they live in a low-resource or high-resource country. The good news is that these children only need temporary pacing after a surgery. In about seven days or so, most patients’ hearts will self-repair. But those seven days are absolutely critical. Now, we can place this tiny pacemaker on a child’s heart and stimulate it with a soft, gentle, wearable device. And no additional surgery is necessary to remove it.”
Although this remarkable new pacemaker represents a significant advancement in treating congenital heart defects, for my daughter as a neonate, her complications were structural, leading to breathing problems, not arrhythmias. It is encouraging to know, however, for the many families with children with CHD that my wife and I met and commiserated with back in the 1980s, that parents in the future will see their children served by groundbreaking devices like this.