July 6, 2014 – If there is one area of engineering pursuit that appears to be inventing all types of new technologies it is the area of energy storage. The most recent innovation comes from University of Southern California (USC) where scientists have been working on an organic battery that contains no metals or toxic materials and can be used in association with power plants.
There are two Achilles Heels to renewable energy form wind and solar. The first is constant availability. The wind doesn’t always blow and the Sun doesn’t always shine. The second is the variability that occurs as winds rise and fall and the Sun angle alters throughout the day impacting photovoltaic energy conversion efficiency.
Innovative solutions have been proposed to deal with these challenges. For example during maximum generation from a renewable energy power plant, electricity not needed for the grid can be used to feed a variety of storage media. The energy can pump water up a hill to create latent energy storage. The water can then be released to flow downhill and turn hydroelectric turbines attached to generators. Or energy not used for power generation can pump air into pressurized cavities or buildings. When released the compressed air can drive wind turbines attached to generators. Both solutions require considerable real estate.
A far more likely technology innovation comes in the form of batteries. We all know about lithium-ion and I have recently written about aluminum-air batteries. But for a technology robust enough to be used to supplement renewable energy power plants we need big batteries with lots of capacity. This is the technology that Sri Narayan, a professor of chemistry at Dornsife College of Letters, Arts and Sciences, USC, describes in a paper published in the Journal of the Electrochemical Society last month. He along with a team of researchers have collaborated in developing an organic-flow battery capable of being recharged through 5,000 cycles for an estimated shelf life of 15 years. That’s 500% more enduring than comparable lithium-ion technology.
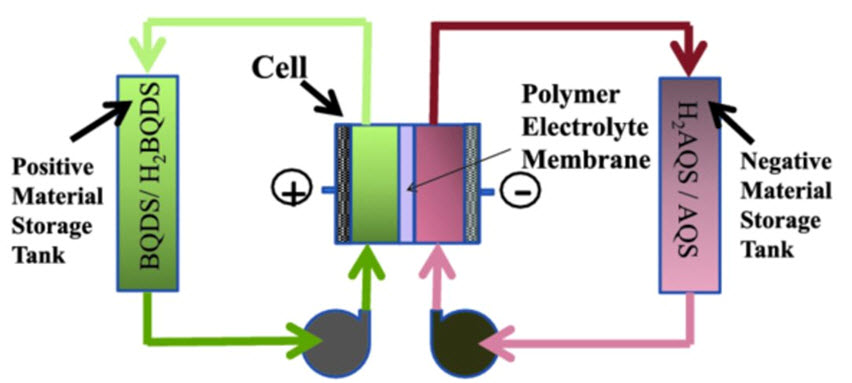
The batteries work similarly to fuel cells. The diagram below displays a schematic of how these batteries work. Two tanks of water containing organic electro-active materials, one solution positive, the other negative, are pumped into a central tank divided by a membrane. The chemicals interact across the membrane release electricity. In the lab the battery is small. But the researchers are confident the battery can go from small to gigantic. And the energy released can also be adjusted to match power demand requirements.
Designs like this are popping up in laboratories in universities in lots of places. The big breakthrough for USC, however, comes in the chemicals. Narayan and his team are using oxidized organic compounds commonly found in plants, fungi, bacteria and animals. These compounds are natural energy-producing chemicals used by animals in respiration and plants for photosynthesis. Called quinones, you may recognize one of the products created from them – hydrogen peroxide. The quinones in use here have been manufactured by the USC team. But in the future the team believes carbon dioxide will be the source for its quinones.
Although still in the lab, the promise of this technology has attracted the U.S. Advanced Research Projects Agency which has provided funding for scaling the battery to build larger versions. If successful this may prove to be a technology present at almost every renewable energy power facility. And we may benefit further from this technology as it helps us reduce our carbon footprint by using captured carbon dioxide for manufacturing the chemicals.











[…] Organic-flow battery could be game-changer for electrical energy storage in association with wind, solar and other renewable power sources. […]