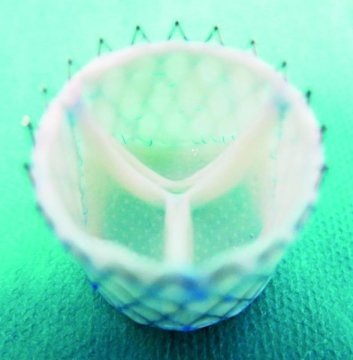
June 29, 2018 – Medical researchers at the University of Zurich, Technical University Eindhoven, and the Charité Berlin are implanting heart valves grown from living cells into sheep in a first step towards making this type of regenerative medicine possible for humans. The heart valves being used are cultured from human cells with the valves being computer designed to be a custom fit.
The effort is part of the European Union-funded LifeValve project, aimed at replacing mechanical and preserved animal tissue valves which are the current standard. The medical team cultures the heart valves from human cells. Design of the valves is done using computer simulations based on detailed imaging of a patient’s heart and outflow tracts to ensure that what is regenerated can be implanted without fitting problems.
Initially, the team used computer simulations to help understand how to culture human cells and grow them into a heart valve. They chose sheep for their animal models and were able over time to come up with accurate bio-engineered valves that could be implanted successfully. And now they have done it with the first successful customized implants in living sheep.
Why is this development so significant?
On a personal note, my daughter was born with a complex congenital heart defect called Tetralogy of Fallot with Absent Pulmonary Valve. She has had two open-heart operations and a third procedure where a valve was inserted into her pulmonary artery via a catheter. All three valves were cryogenically preserved dead animal valve tissue. My daughter faces a lifetime of these periodic valve replacements because dead tissue cannot regenerate itself.
Worldwide the incidence of congenital (meaning born with) heart disease (CHD) is 8 to 12 for every one thousand live births, amounting to over 1.3 million globally each year. This worldwide phenomenon knows no boundaries occurring in populations everywhere with only minor variation by country in CHD type.
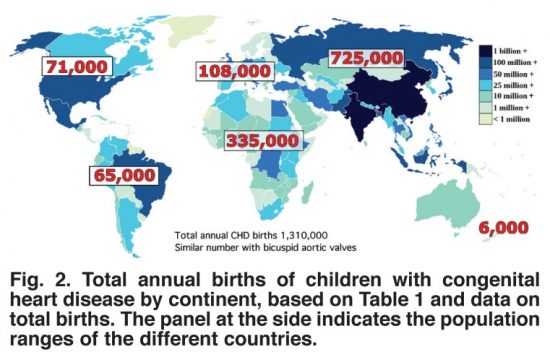
Structural repairs for most CHDs involves patching holes in the heart, altering arterial structures to improve blood flow either through the lungs or to the bodies extremities, and replacing missing or damaged valves. The human heart has four and depending on the type of CHD, any one or more of these valves could require a replacement implant.
With current practice using dead animal tissue valves, CHD patients undergo repeated surgical interventions throughout their lives. That’s because the valve flaps wear out, or calcify to become less flexible and restrict arterial blood flow. The alternative surgical prosthetic, mechanical valve implants, may last a lifetime, but require CHD patients to stay continuously on anticoagulants to prevent blood clots. But growing a heart valve with the CHD patient’s own human cells would eliminate the problems attendant to both current practices.
The biggest challenge is no longer in the growing but in the custom fitting of what is grown. There is no one size fits all solution. But it appears that the work being done in Zurich may soon be reflected in the first human clinical trials giving children born with congenital heart disease a much better life requiring fewer surgical interventions. Eventually, the bio-engineering of heart tissue using a patient’s own cells could heal the hearts of all children with CHD.
The results from Zurich have been published in the journal, Science Translational Medicine, May 9, 2018 issue, in an article entitled, “Computational modeling guides tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model.”