What are smart antibiotics? They are disease-selective drugs that target a specific pathogen that causes disease while not harming beneficial bacteria in our gut. They target harmful bacteria usually by using a synthetic or bacteriophage (virus) carrier to penetrate the cell barrier and release its lethal dose to dispatch the pathogen. Because they are highly specific, the side effects from their administration are significantly less.
The challenge in their use is bacteria rapidly evolve to render smart antibiotics ineffective meaning a continuous arms race exists between developers of smart drugs and the pathogens they target. That’s why the vast number of antibiotics in general use are not bacteria-specific and cause casualties for the beneficial ones our bodies host and need.
At the University of Basel in Switzerland, scientists have been developing an antibiotic designed to kill gram-negative bacteria also known as superbugs like Escherichia coli (E-coli) and Klebsiella pneumoniae.
E-coli causes urinary tract, enteric and, in some cases diseases like hemolytic uraemic syndrome (HUS) which is fatal in 3 to 5% of cases. E-coli can also cause neurological complications including seizures, strokes and even coma.
Klebsiella pneumonia, as you might suspect by its name, causes pneumonia. It also, however, can cause urinary, blood, and wound infections. It is particularly dangerous to those admitted into hospital for other reasons, being the most common cause of hospital-acquired infections (HAIs).
Non-specific antibiotics when used to treat antibiotic-resistant pathogens cause complications. When good bacteria get killed by the antibiotic, pathogens like Clostridiodes difficile (C-difficile) can bloom causing bowel inflammatory disease and sepsis.
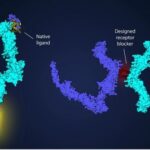
The Basel research has led to the development of an antibiotic called Lolamicin. It doesn’t directly target the pathogen. Instead, it includes compounds that inhibit the transport of pathogen lipoproteins that use the LoL (lipopolysaccharide ligase) system. For example, E-coli uses the LoL to penetrate cell barriers. Lolamicin has demonstrated it can inhibit this transport effectively causing the pathogen to die. At the same time, the antibiotic leaves other bacteria alone.
So far Lolamicin has killed more than 130 multidrug-resistant strains of bacteria when grown in the laboratory. In mouse studies, those exposed to antibiotic-resistant bacteria have all survived after being given Lolamicin. A control group, on the other hand, experienced an 87% mortality rate within three days. The mice given Lolamicin saw no loss of healthy gut bacteria and no appearance of C-difficile. Other mice given Amoxicillin, however, did experience C-difficile infections attributed to the loss of beneficial gut bacteria.
Mouse studies aren’t human studies. We remain a long way off before we see Lolamicin or a successor used to treat humans. Novel antibiotics like Lolamicin, however, tend to be forgotten by big pharmaceutical companies. Between ten and twenty developed in the last decade have still not received approval from the United States Food and Drug Administration (FDA).
Considering the growing incidents of E-coli, Klebsiella pneumoniae, and C-difficile in the general population, you would think the pharmaceutical industry would be very interested in novel antibiotics. When I did a quick survey of the medical literature, I found some disturbing studies that support big pharma becoming more involved.
For example, a Calgary Health District study reported attempts to treat E-coli and noted growing resistance to ampicillin, trimethoprim-sulphamethoxazole, gentamicin, ciprofloxacin, cefazolin and ceftriaxone with a case-fatality rate of 11%.
A similar Calgary-based study treating Klebsiella pneumoniae showed growing resistance to trimethoprim/sulfamethoxazole and case-fatality rates of 20%.
A U.S. hospital study reported that C-difficile is increasingly placing a large burden on hospitals extending stays for patients who develop HAIs after admission for unrelated medical conditions and treatment.
If you are interested in learning more about Lolamicin, the University of Basel team recently published their work in the Journal Nature.