One of my readers has challenged me to find evidence of ocean acidification on zooplankton and other sea life having pointed out that the studies I have cited included freshwater results or are purely in laboratories. That’s why I have chosen to highlight a story that appeared this week in the Los Angeles Times and in BBC Nature News.
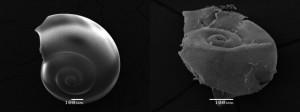
Both refer to a Nature Geoscience article published on November 25, 2012, entitled, Extensive dissolution of live pteropods in the Southern Ocean. The article is the published results of an in the wild study that looked at anthropogenic, that is human influenced, levels of CO2 and its impact on shelled marine creatures in the waters off Antarctica. The research data described was compiled from studying acidification levels and calcium carbonate levels in pteropods, marine snails, inhabiting the top 200 meters of a water column in the Southern Ocean. Data was collected in 2008 and concluded that these particular sea creatures were experiencing decalcification showing up as shell corrosion. The importance of studying pteropods is their place at the base of the ocean food chain and the potential impact their destruction could have on fish, seabird and whale populations.

The measurements looked at samples from cold seawater at depths of as much as 1,000 meters (3,300 feet) upwelling to the surface. Aragonite, a form of calcium carbonate that is found in pteropod shells, was measured. The researchers hypothesized that the combined effect of the natural upwelling of cold water with higher levels of CO2 was creating an acidity saturation horizon at depths from 200 meters to the surface where the pteropods thrive. That combination of the cold water and acidity appeared to be having a much higher corrosive impact on shelled creatures than anything observed in warmer waters.
You probably are asking yourselves what does cold water upwelling have to do with anthropogenic CO2 acidification? When deep cold ocean water rises to the surface and mixes with the more acidic shallow water, it cannot compensate for the cold water resulting in amplified acidification.
Who was involved and What did they Conclude?
Scientists from the British Antarctic Survey, the U.S. National Oceanic and Atmospheric Administration (NOAA), the Woods Hole Oceanographic Institution, and the university of East Anglia’s School of Environmental Sciences.
Their conclusions:
That ocean acidification from increased absorption of CO2 (correlated to human causes such as burning of fossil fuels) was the likely causal agent.
This is the first open ocean study looking at acidification and its impact on decalcification of a particular species in the ocean. Up until now all studies have been in laboratory settings. Described as a pilot project, the researchers intend to do further in the wild studies looking beyond pteropods to assess the impact of increased acidity on other ocean life.
Similar research is being conducted by scientists at the University of New South Wales in Australia who report that rising acid levels in the Southern Oceans will have a destructive impact on sea life within the next thirty years.
What ocean life is most vulnerable? Cold water coral communities, the nursery habitat for hundreds of commercial fish species, are probably most vulnerable, as are molluscs, starfish and sea urchins. In the community that constitutes phytoplankton, besides pteropods, we can expect similar decalcification impacts on foraminifera (single-celled amoeboid-like creatures with chalky shells) and coccolithophores (single-celled algae). And although shell corrosion does not necessarily mean the death of sea creatures that rely on calcium carbonate, any alteration in calcification rates that causes structural damage to organisms cannot be a good sign.










My “challenge” was related to the 40% Phytoplankton decline reported in Nature a couple of years back. The true base of the oceanic food chain is Phytoplankton, not zooplankton. While the zooplankton report you posted is mildly interesting, and not exactly conclusive, it seems to have no bearing on the phytoplankton questions. Where is the study that shows “acidification” or warming surface waters is causing the phytoplankton decline? For all I know from the data I’ve seen, the phytoplankton decline might just as well be largely responsible for the warming surface waters and rising CO2 levels as the reverse.
In the study although they didn’t focus on phytoplankton they reference the susceptibility of coccolithophores.
Look at the Wiki Coccolithophores article: http://en.wikipedia.org/wiki/Coccolithophore
“Coccolithophores have long been thought to respond to increased ocean acidity, caused by increasing CO2 levels, by becoming less calcified. In 2008, Iglesias-Rodriguez et al. were surprised to learn that in fact the opposite can happen in at least some circumstances, with the model species E. huxleyi becoming 40% heavier and more abundant in waters of higher CO2 concentration.”
Sorry, ambiguous and conflicting evidence fails to persuade.
I don’t pretend to know what is causing the phytoplankton decline. I’m still waiting for a definitive study by someone who does know. I’m guessing no one knows, although there are many who offer speculations as evidence.
Just as availability of photosynthesized food (grass), not population and biomass of predators, determines African veldt population and biomass of prey species, the same would mostly hold true for marine animals, which are utterly dependent on the phytoplankton as the broad base of the oceanic food pyramid. Sure mankind can over-fish, and temporarily crash populations of some species, but as long as the phytoplankton holds up in good health and abundance, most over-fished species would promptly rebound once aggressive harvesting pressures are relieved. But over-fished species won’t rebound without abundant phytoplankton.
Falling fish populations coupled with falling phytoplankton levels creates a non-deterministic process with many potential bifurcation points. See: “Turbulent Mirror.” The 29 July 2010 “Nature” article warned we have already lost 40% of the phytoplankton, and we continue to lose an additional 1% per year. That’s not just a cause for mild concern; that’s cause for major alarm. It’s particularly alarming because we don’t really know what is causing the decline. For all we know, reducing atmospheric CO2 levels might hasten the phytoplankton decline.
Evaporation pan studies have shown that atmospheric soot and aerosols are slowing global greenhouse warming perhaps by as much as 8-10 degrees C. The particulates are acting as an opaque umbrella retarding surface insolation. See: http://www.mindfully.org/Air/2002/Decreased-Pan-Evaporation1nov02.htm
So how about this scene? Stop burning coal and watch average global temperature rise maybe 10 degrees. How would that affect the rate of Arctic methane hydrate breakdown? How much temperature rise would it take to trigger a positive feedback avalanche methane release? This would be a really smart time to implement well-funded crash programs to discover what is actually causing the phytoplankton decline. I’m guessing mankind will not like the honest answers because the bottom line will come down to, “mankind must drastically reduce its population levels.” If humanity will not devise reasonable means of population reduction, then nature will apply indifferent means.
What government has the courage to tell its constituents they can no longer breed? What government seeking tax revenues wants to deal with the shortfalls that would be produced by a population decline? This is the Achilles Heel of our unwillingness to address human population growth. And to make matters worse, the population bomb isn’t happening in places where we are relatively well off. It is happening in the poorest of countries. And that’s because we have improved medical services to these places to eliminate high infancy mortality. But what we haven’t done is solve many of the other problems that are inherently a part of the post-colonial legacy.
I’ll continue to look for phytoplankton studies because I don’t always trust what can be found in Wikipedia. Your point, however, about the dearth of good studies is disturbing. And yet our leaders meet in the Persian Gulf and dither on the issues that will determine the future health of the planet.
<>
Unless the truth directly relates to the preservation of government and enrichment of its principal ministers, what government has the courage to tell its constituents any unwelcome truth at all? History is not well supplied with good examples of instances where governments acted in the interests of their constituents and at the expense of “the “governments” and their ministers. As Sun Tzu correctly taught us thousands of years ago, the art of war is also the science of deceit. Why pay for desired objectives with blood and gold when lying, cheating, theft, extortion, torture, and outright murder more cheaply obtain them? That dark lesson is not lost on governments. Everyone I know thinks the successful politician is mostly a successful liar and cheat. How much have things really changed since Tacitus gave us his cynical and dismal, “Annals of Imperial Rome”? What did Machiavelli get wrong in his “Discourses on Livy”? What key conception was it that Le Boetie misunderstood or omitted in his, “On Voluntary Servitude” treatise?
The great statesman is he who achieves personal self-enrichment while at the same time serving the public interest, even when the public resents and resists his efforts. That’s the most we can hope for. As the Hitlerian Nazi’s were plotting the political destruction of Christian Civilization and military conquest of all of Europe, Churchill said the unwelcome truth of it, that the world would have to pay a high price to stop the Nazis now, or else the price would soon be much higher, and perhaps beyond the free world’s capacity to pay. Neville Chamberlain’s “peace in our time” cheap appeasement message was much more welcome. Perhaps it could have been no other way. Churchill didn’t become wartime prime minister after Chamberlain’s resignation due to popular support and acclaim, it was mostly because after the fall or Norway, all the leading politicians had serious doubts that the war with Germany could be won, and didn’t seek the post. Churchill himself shared those doubts. It was not honest courage and ingenuity of politicians that defeated the evils of Nazi and imperial Japanese conquest. It was pure dumb luck.
The key constituency of our modern industrial “democracy” states consists mostly of unproductive vampires that are nourished by the stolen blood of unborn generations and the insurance of the governments’ “full faith and credit.” Are we now to watch our rich global civilization destroy itself because the constituencies of obscene vampires will hate those who diminish their blood supply?
Modern politics has little to do with statesmanship. Who in his right mind supposes national governments, all with conflicts of interest with each other, will implement effective carbon cap and trade programs that preserve and restore the global phytoplankton, especially considering that the main physical causes of the decline are unknown? Governments are behaving like addled-brained drunks searching for lost keys under the streetlights because that’s where most of the light is. The drunks don’t want to know exactly where the keys were lost. They want to make a big show out of searching where they will never find them.
Pardon me if I refuse to join the drunken key search before some rational case is made for where the keys were actually lost.
I fully appreciate your cynical view of government. But I do see a potential for change in our present world as we begin to see citizens’ groups unite around causes of consequence, and start driving an agenda that no government would be willing to present. Yes we do have imperfect models, and the science of understanding the data we are collecting remains imperfect, but that isn’t stopping movements like 350.org and others from marshaling people to challenge those that rule to implement policies that may lead to where you don’t believe we can ever go.
The issue of politics and statesmanship is interesting. Was Lincoln a politician or a statesman? Was FDR? Was Churchill? I believe these three knew how to play the political game but they pushed agendas that achieved survival against all odds.
((The issue of politics and statesmanship is interesting. Was Lincoln a politician or a statesman? Was FDR? Was Churchill? I believe these three knew how to play the political game but they pushed agendas that achieved survival against all odds.))
All statesmen are politicians; only rarely are politicians also statesmen. Lincoln, FDR, and Churchill were all statesmen.