June 15, 2015 – What is “designer carbon?” What’s it good for? According to Stanford researchers – lots of applications including energy storage, solar panels and even carbon capture.
Heading up the Stanford team is Zhenan Bao, Professor of Chemical Engineering. She and his colleagues have published their “designer carbon” discovery, which they call HPG carbon, in the most recent issue of the journal ACS Central Science. Activated carbon is often produced from coconut shells but the materials contain pores with no consistency. Her team found a new method of synthesizing carbon using a water-based polymer similar to the material in a soft contact lens. These polymers are classified as hydrogels and form a three-dimensional interconnected network containing organic molecules and nitrogen. The material is ideal for conducting electricity.
The team manufactured nanometer-thick carbon sheets and then added potassium hydroxide to increase their surface areas. Voila, designer carbon which can be altered chemically by changing the polymer and organic composition and by applying heat to increase pore volume. This creates carbon with record-high surface areas – over 4,000 square meters per gram making the material useful for numerous applications.
The design team sees applications for super-capacitors, batteries and electrodes. One application should produce lithium-sulfur batteries that are stable and perform better. With the material heated to create a highly dense mat of small pores you don’t have any polysulfides leaching out to weaken battery performance.
And probably the best feature of all for HPG carbon is that it’s simple to make in a one-step process and relatively inexpensive. In the journal paper, the authors state “our synthetic method…allows easy incorporation of metal, metal oxides, nitrides, or carbides in the carbon framework by adding metal-containing salts during the polymerization process.” It is this fine “tunablility” that makes HPG carbon adaptable for a wide range of applications.
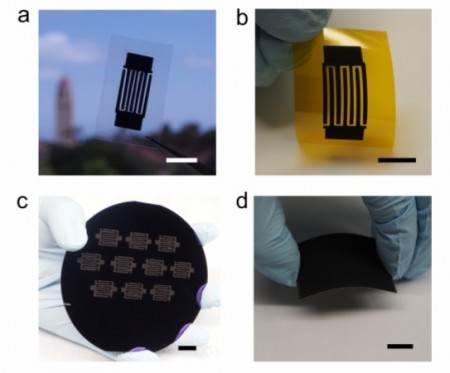
To give you an idea the team produced a number of electrodes and supercapacitors. They can be seen in the picture below.
“A” is an interdigital supercapacitor made with designer carbon ink on gold-coated film. “B” is a flexible supercapacitor with interdigital electrodes made with designer carbon spray applied to polymide film. “C” is a silicon wafer fabricated with 10 supercapacitors made from designer carbon, and “D” is an electrode 100 nanometers thick applied to a flexible substrate.
The research was funded in part by the Global Climate Energy Project, Preourt Institute for Energy, United States Department of Energy, Battery Materials Research Program and the SUNCAT Center for Interface Science and Catalysis.